BSS
Alcon Laboratories, Inc.
Alcon Laboratories, Inc.
(balanced salt solution)
FULL PRESCRIBING INFORMATION: CONTENTS*
- BSS DESCRIPTION
- CLINICAL PHARMACOLOGY
- BSS INDICATIONS AND USAGE
- WARNINGS
- PRECAUTIONS
- BSS ADVERSE REACTIONS
- BSS DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
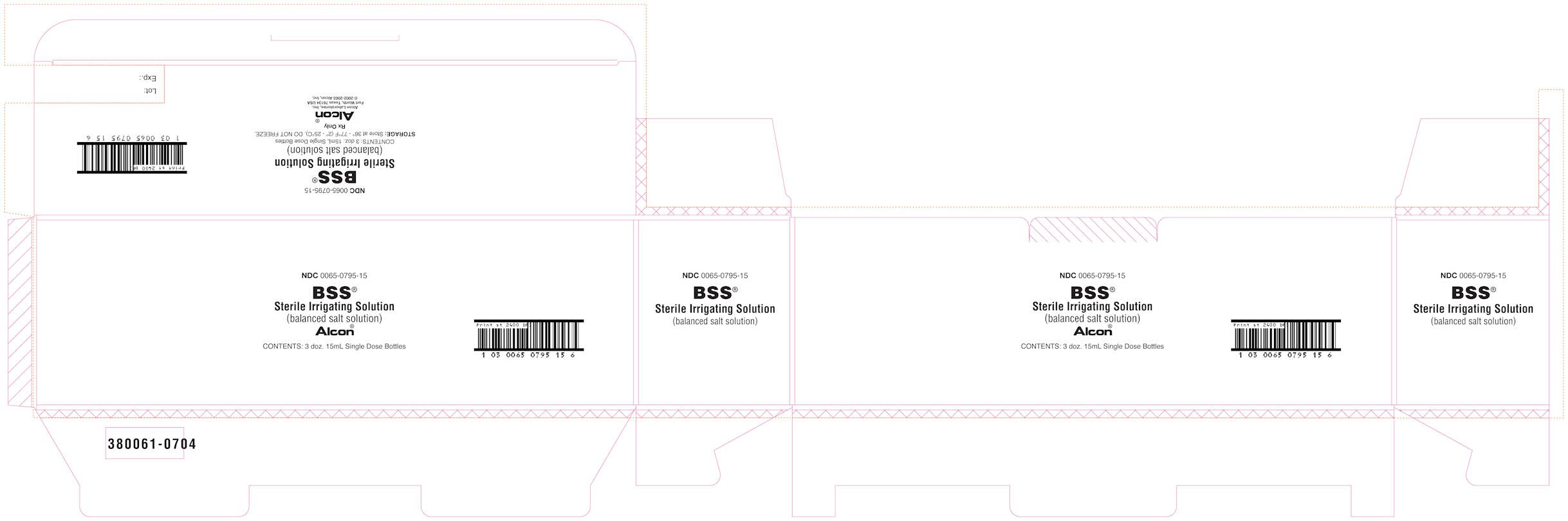
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
DESCRIPTION
BSS® Sterile Irrigating Solution is a sterile balanced salt solution, each mL containing sodium chloride (NaCl) 0.64%, potassium chloride (KCl) 0.075%, calcium chloride dihydrate (CaCl2•2H2O) 0.048%, magnesium chloride hexahydrate (MgCl2•6H2O) 0.03%, sodium acetate trihydrate (C2H3NaO2•3H2O) 0.39%, sodium citrate dihydrate (C6H5Na3O7•2H2O) 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection. The pH is approximately 7.5. The osmolality is approximately 300 mOsm/Kg.
CLINICAL PHARMACOLOGY
BSS Sterile Irrigating Solution is an isotonic solution for use in irrigating tissues of the eyes.
INDICATIONS AND USAGE
For use as an extraocular and intraocular irrigating solution during ocular surgical procedure involving perfusion of the eye with an expected maximum duration of less than 60 minutes.
WARNINGS
• NOT FOR INJECTION OR INTRAVENOUS INFUSION.
• Do not use unless product is clear, seal is intact, and container is undamaged.
• Do not use if product is discolored or contains a precipitate.
• SINGLE patient use only. The contents of this bottle should not be used in more than one patient.
• The use of additives with this solution may cause corneal decompensation.
• This solution contains no preservative, unused contents should be discarded.
PRECAUTIONS
Open under aseptic conditions only.
Prior to use, check the following: tip should be firmly in place, irrigating needle should be properly seated; squeeze out several drops before inserting into anterior chamber. The needle should be removed from the anterior chamber prior to releasing pressure to prevent suction.
Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding or edema following ocular surgery in which BSS Sterile Irrigating Solution was used as an irrigating solution.
ADVERSE REACTIONS
Irrigation or any other trauma to the corneal endothelium may result in corneal swelling or bullous keratopathy. Postoperative inflammatory reactions as well as incidents of corneal edema and corneal decompensation have been reported.
DOSAGE AND ADMINISTRATION
The adapter plug is designed to accept an irrigating needle. Tissues may be irrigated by attaching the needle to the DROP-TAINER® bottle as explained below. External irrigation may be done without the irrigating needle.
Method of using Adapter Plug for LUER-LOK* Hub Ophthalmic Irrigating Needle:
1. Aseptically remove DROP-TAINER bottle from blister by peeling paper backing.
2. Snap on surgeon's sterile irrigation needle. Push until firmly in place and twist slightly.
3. Test assembly for proper function before use.
*NOTE: LUER-LOK is a registered trademark of Becton, Dickinson and Company.
HOW SUPPLIED
In 15 mL and 30 mL sterile DROP-TAINER® bottles (consisting of a polypropylene bottle and LEUR-LOK* adapter plug with a white polypropylene closure) in a blister package.
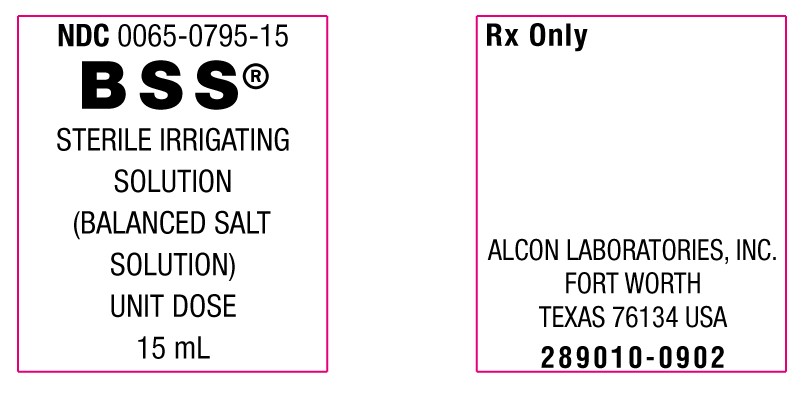
15 mL in a 15 mL bottle: NDC 0065-0795-15
30 mL in a 30 mL bottle: NDC 0065-0795-30
STORAGE: Store at 36° - 77°F (2° - 25°C). DO NOT FREEZE.
Rx Only
Revised: December 2003
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, Texas 76134 USA
Printed in USA
©2000-2003 Alcon, Inc.
340062-0704
PRINCIPAL DISPLAY PANEL
NDC 0065-0795-15
BSS®
Sterile Irrigation Solution
(balanced salt solution)
Alcon®
Contents: 3 doz. 15mL Single Dose Bottles

BSSbalanced salt solution SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||