Zolpidem Tartrate
RedPharm Drug, Inc.
Aurobindo Pharma Limited
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use zolpidem tartrate safely and effectively. See full prescribing information for zolpidem tartrate tablets.Zolpidem Tartrate Tablets CIVInitial U.S. Approval: 1992 RECENT MAJOR CHANGES1Warnings and Precautions5.25.35.6INDICATIONS AND USAGE(1)DOSAGE AND ADMINISTRATION Adult dose: 10 mg once daily immediately before bedtime (2.1) Elderly/debilitated patients/hepatically impaired: 5 mg once daily immediately before bedtime (2.2) Downward dosage adjustment may be necessary when used with CNS depressants (2.3) Should not be taken with or immediately after a meal (2.4) DOSAGE FORMS AND STRENGTHSnot(3)CONTRAINDICATIONS(4)WARNINGS AND PRECAUTIONS Need to evaluate for co-morbid diagnosis: Reevaluate if insomnia persists after 7 to 10 days of use (5.1) Severe anaphylactic/anaphylactoid reactions: Angioedema and anaphylaxis have been reported. Do not rechallenge if such reactions occur. (5.2) Abnormal thinking, behavioral changes and complex behaviors: May include “sleep-driving” and hallucinations. Immediately evaluate any new onset behavioral changes. (5.3) Depression: Worsening of depression or, suicidal thinking may occur. Prescribe the least amount feasible to avoid intentional overdose (5.3, 5.6) Withdrawal effects: Symptoms may occur with rapid dose reduction or discontinuation (5.4, 9.3) CNS depressant effects: Use can impair alertness and motor coordination. If used in combination with other CNS depressants, dose reductions may be needed due to additive effects. Do not use with alcohol (2.3, 5.5) Elderly/debilitated patients: Use lower dose due to impaired motor, cognitive performance and increased sensitivity (2.2, 5.6) Patients with hepatic impairment, mild to moderate COPD, impaired drug metabolism or hemodynamic responses, mild to moderate sleep apnea: Use with caution and monitor closely. (5.6) Side Effects6.1 To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS CNS depressants: Enhanced CNS-depressant effects with combination use. Use with alcohol causes additive psychomotor impairment (7.1) Imipramine: Decreased alertness observed with combination use. (7.1) Chlorpromazine: Impaired alertness and psychomotor performance observed with combination use. (7.1) Rifampin: Combination use decreases exposure to and effects of zolpidem (7.2) Ketoconazole: Combination use increases exposure to and effect of zolpidem (7.2) USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, zolpidem may cause fetal harm. ( 8.1) Nursing mothers: Zolpidem is excreted in human milk. (8.3) Pediatric use: Safety and effectiveness not established. Hallucinations (incidence rate 7.4%) and other psychiatric and/or nervous system adverse reactions were observed frequently in a study of pediatric patients with Attention-Deficit/Hyperactivity Disorder (5.6, 8.4)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ZOLPIDEM TARTRATE INDICATIONS AND USAGE
- 2 ZOLPIDEM TARTRATE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ZOLPIDEM TARTRATE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ZOLPIDEM TARTRATE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 ZOLPIDEM TARTRATE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- 17.4 Medication Guide
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
[see Clinical Studies (14)]
2 DOSAGE AND ADMINISTRATION
The dose of zolpidem tartrate tablets should be individualized.
2.1 Dosage in adults
2.2 Special populations
[see Warnings and Precautions (5.6)].
2.3 Use with CNS depressants
[see Warnings and Precautions (5.5)].
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
[see Warnings and Precautions (5.2)]
5 WARNINGS AND PRECAUTIONS
5.1 Need to evaluate for co-morbid diagnoses
The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated.
5.2 Severe anaphylactic and anaphylactoid reactions
5.3 Abnormal thinking and behavioral changes
[see Use in Specific Populations (8.4)].
5.4 Withdrawal effects
[see Drug Abuse and Dependence (9)].
5.5 CNS depressant effects
Due to the rapid onset of action, zolpidem tartrate should only be taken immediately prior to going to bed.
5.6 Special populations
Use in the elderly and/or debilitated patients: [see Dosage and Administration (2.2)].
Use in patients with concomitant illness:
[see Clinical Pharmacology (12.3)].
[see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
Use in patients with depression:
Use in pediatric patients: [see Use in Specific Populations (8.4)].
6 ADVERSE REACTIONS
- Serious anaphylactic and anaphylactoid reactions [see Warnings and Precautions (5.2)]
- Abnormal thinking, behavior changes, and complex behaviors [see Warnings and Precautions (5.3)]
- Withdrawal effects [see Warnings and Precautions (5.4)]
- CNS-depressant effects [see Warnings and Precautions (5.5)]
6.1 Clinical trials experience
Associated with discontinuation of treatment:
Most commonly observed adverse reactions in controlled trials:
Adverse reactions observed at an incidence of ≥ 1% in controlled trials:
| Body System/Adverse Event* | Zolpidem (≤10 mg) (N=685) |
Placebo (N=473) |
|---|---|---|
| * Reactions reported by at least 1% of patients treated with zolpidem tartrate and at a greater frequency than placebo. |
||
| Central and Peripheral Nervous System |
||
| Headache |
7 |
6 |
| Drowsiness |
2 |
- |
| Dizziness |
1 |
- |
| Gastrointestinal System |
||
| Diarrhea |
1 |
- |
| Body System/Adverse Event* | Zolpidem (≤10 mg) (N=152) |
Placebo (N=161) |
|---|---|---|
| * Reactions reported by at least 1% of patients treated with zolpidem tartrate and at a greater frequency than placebo. |
||
| Autonomic Nervous System |
||
| Dry mouth |
3 |
1 |
| Body as a Whole |
||
| Allergy |
4 |
1 |
| Back Pain |
3 |
2 |
| Influenza-like symptoms |
2 |
- |
| Chest pain |
1 |
- |
| Cardiovascular System |
|
|
| Palpitation |
2 |
- |
| Central and Peripheral Nervous System |
|
|
| Drowsiness |
8 |
5 |
| Dizziness |
5 |
1 |
| Lethargy |
3 |
1 |
| Drugged feeling |
3 |
- |
| Lightheadedness |
2 |
1 |
| Depression |
2 |
1 |
| Abnormal dreams |
1 |
- |
| Amnesia |
1 |
- |
| Sleep disorder |
1 |
- |
| Gastrointestinal System |
||
| Diarrhea |
3 |
2 |
| Abdominal pain |
2 |
2 |
| Constipation |
2 |
1 |
| Respiratory System |
||
| Sinusitis |
4 |
2 |
| Pharyngitis |
3 |
1 |
| Skin and Appendages |
||
| Rash |
2 |
1 |
Adverse event incidence across the entire preapproval database:
Autonomic nervous system:
Body as a whole:
Cardiovascular system:
Central and peripheral nervous system:
Gastrointestinal system:
Hematologic and lymphatic system: Rare: anemia, hyperhemoglobinemia, leukopenia, lymphadenopathy, macrocytic anemia, purpura, thrombosis.
Immunologic system: Infrequent: infection. Rare: abscess herpes simplex herpes zoster, otitis externa, otitis media.
Liver and biliary system:
Metabolic and nutritional:
Musculoskeletal system:
Reproductive system:
Respiratory system:
Skin and appendages:
Special senses:
Urogenital system:
7 DRUG INTERACTIONS
7.1 CNS-active drugs
[see Warnings and Precautions (5.5)].
maxmax
7.2 Drugs that affect drug metabolism via cytochrome P450
0-∞
max1/2
max
7.3 Other drugs with no interaction with zolpidem
7.4 Drug-laboratory test interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
2 2 2 2 2 2
Neonatal Complications
8.2 Labor and delivery
[see Pregnancy (8.1)].
8.3 Nursing mothers
8.4 Pediatric use
[see Warnings and Precautions (5.6)].
8.5 Geriatric use
| Adverse Event | Zolpidem | Placebo |
|---|---|---|
| Dizziness |
3% |
0% |
| Drowsiness |
5% |
2% |
| Diarrhea |
3% |
1% |
[see Warnings and Precautions (5.6)]
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Signs and symptoms
10.2 Recommended treatment
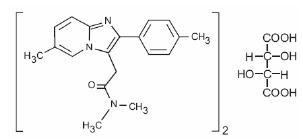
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
in vitro1 15 1
12.3 Pharmacokinetics
1/2
maxmax
maxmax
Special Populations
Elderly
[see Warnings and Precautions (5) and Dosage and Administration (2)]max1/2max1/2
Hepatic Impairment
max max[see Dosage and Administration (2.2) and Warnings and Precautions (5.6)]
Renal Impairment
Crmaxmaxmaxmaxmaxmax1/21/2
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, mutagenesis, impairment of fertility
Carcinogenesis2 2
Mutagenesisin vitro in vivo
Impairment of fertility2 2
14 CLINICAL STUDIES
14.1 Transient insomnia
14.2 Chronic insomnia
14.3 Studies pertinent to safety concerns for sedatives/hypnotic drugs
Next-day residual effects:
Rebound effects:
Memory impairment:
Effects on sleep stages:
16 HOW SUPPLIED/STORAGE AND HANDLING
Zolpidem Tartrate Tablets, 5 mg
Zolpidem Tartrate Tablets, 10 mg
Store at
17 PATIENT COUNSELING INFORMATION
[see Medication Guide (17.4)]
17.1 Severe anaphylactic and anaphylactoid reactions
17.2 Sleep-driving and other complex behaviors
[see Warnings and Precautions (5.3)]
17.3 Administration instructions
Patients should be counseled to take zolpidem tartrate right before they get into bed and only when they are able to stay in bed a full night (7 to 8 hours) before being active again. Zolpidem tartrate tablets should not be taken with or immediately after a meal. Advise patients NOT to take zolpidem tartrate when drinking alcohol.
17.4 Medication Guide
Zolpidem Tartrate Tablets CIV
Rx only
What is the most important information I should know about zolpidem tartrate?
After taking zolpidem tartrate, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night.
- driving a car ("sleep-driving")
- making and eating food
- talking on the phone
- having sex
- sleep-walking
Call your doctor right away if you find out that you have done any of the above activities after taking zolpidem tartrate.
Important:
-
Take zolpidem tartrate exactly as prescribed
- Do not take more zolpidem tartrate than prescribed.
- Take zolpidem tartrate right before you get in bed, not sooner.
-
Do not take zolpidem
tartrate if you:
- drink alcohol
- take other medicines that can make you sleepy. Talk to your doctor about all of your medicines. Your doctor will tell you if you can take zolpidem tartrate with your other medicines.
- cannot get a full night’s sleep
What is zolpidem tartrate?
- trouble falling asleep
Who should not take zolpidem tartrate?
Zolpidem tartrate may not be right for you. Before starting zolpidem tartrate, tell your doctor about all of your health conditions, including if you:
- have a history of depression, mental illness, or suicidal thoughts
- have a history of drug or alcohol abuse or addiction
- have kidney or liver disease
- have a lung disease or breathing problems
- are pregnant, planning to become pregnant, or breastfeeding
Do not take zolpidem tartrate with other medicines that can make you sleepy.
How should I take zolpidem tartrate?
- Take zolpidem tartrate exactly as prescribed. Do not take more zolpidem tartrate than prescribed for you.
- Take zolpidem tartrate right before you get into bed.
- Do not take zolpidem tartrate unless you are able to stay in bed a full night (7 to 8 hours) before you must be active again.
- For faster sleep onset, zolpidem tartrate should NOT be taken with or immediately after a meal.
- Call your doctor if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- If you take too much zolpidem tartrate or overdose, call your doctor or poison control center right away, or get emergency treatment.
What are the possible side effects of zolpidem tartrate?
Serious side effects of zolpidem tartrate include:
- getting out of bed while not being fully awake and do an activity that you do not know you are doing. (See “What is the most important information I should know about zolpidem tartrate?”)
- abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, hallucinations, worsening of depression, and suicidal thoughts or actions.
- memory loss
- anxiety
- severe allergic reactions. Symptoms include swelling of the tongue or throat, trouble breathing, and nausea and vomiting. Get emergency medical help if you get these symptoms after taking zolpidem tartrate.
Call your doctor right away if you have any of the above side effects or any other side effects that worry you while using zolpidem tartrate.
The most common side effects of zolpidem tartrate are:
- drowsiness
- dizziness
- diarrhea
- “drugged feelings”
- You may still feel drowsy the next day after taking zolpidem tartrate. Do not drive or do other dangerous activities after taking zolpidem tartrate until you feel fully awake.
After you stop taking a sleep medicine,
How should I store zolpidem tartrate?
- Store zolpidem tartrate at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
- Keep zolpidem tartrate and all medicines out of reach of children.
General Information about zolpidem tartrate
- Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
- Do not use zolpidem tartrate for a condition for which it was not prescribed.
- Do not share zolpidem tartrate with other people, even if you think they have the same symptoms that you have. It may harm them and it is against the law.
What are the ingredients in zolpidem tartrate tablets?
Active Ingredient:
Inactive Ingredients:
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
zolpidem5mg
Zolpidem TartrateZolpidem Tartrate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||