Volumex
Volumex
FULL PRESCRIBING INFORMATION
Rx Only
Volumex (Iodinated I 131 Albumin Injection) is a diagnostic radiopharmaceutical
containing iodinated I 131 albumin for intravenous use. Each mL of sterile,
nonpyrogenic, aqueous, colorless to very pale yellow solution provides approxi-
mately 10 mg protein (albumin human), 16 mg dibasic sodium phosphate, 1.6 mg
monobasic sodium phosphate, not more, than 0.4 mg guanidine hydrochloride,
sodium chloride for isotonicity, and 9 mg benzyl alcohol as a preservative. The
stabilizer acetyltryptophanate and sodium caprylate have a concentration of less
than 0.0089M. The pH has been adjusted to 7.2-7.8 with sodium hydroxide or
hydrochloric acid.
Volumex was prepared from blood that was nonreactive when tested for hepa-
titis B surface antigen (HBsAg). The structure of the complex is unknown.
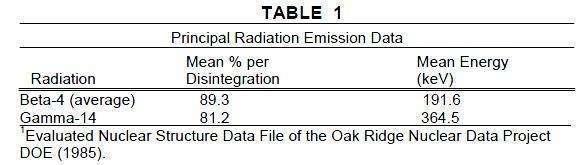
Iodine 131 decays by beta and gamma emissions with a physical half-life of 8.08 days.1
Photons that are useful for detection and imaging studies are listed in Table 1.
EXTERNAL RADIATION
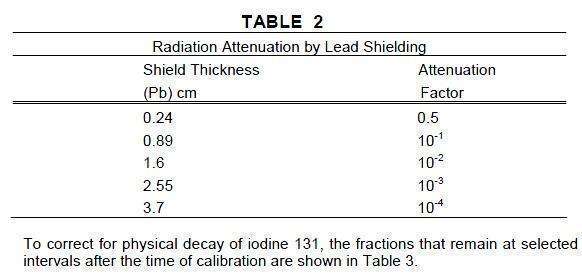
The specific gamma ray constant for iodine 131 is 2.2R/hour-millicurie at 1 cm.
The first half-value layer is 0.24 cm lead (Pb). A range of values for the relative
attenuation of the radiation emitted by this radionuclide that result from interpo-
sition of various thicknesses of Pb is shown in Table 2. To facilitate control of
the radiation exposure from this radionuclide, the use of a 2.55 cm thickness of Pb
will attenuate the radiation emitted by a factor of about 1,000.
Following intravenous injection, radioiodinated albumin human is uniformly dis-
tributed throughout the intravascular pool within 10 minutes; extravascular distri-
bution takes place more slowly. Iodinated I 131 albumin also can be detected in
the lymph and in certain body tissues within 10 minutes after injection, but maxi-
mum distribution of radioactivity throughout the extravascular space does not
occur until two to four days after administration. The time at which extravascular
activity is maximal has been designated as the “equilibrium time.” When this point
has been reached, the radioactivity remaining in the intravascular and extravas-
cular spaces decreases slowly and exponentially in parallel fashion.
The administered radioactivity is eliminated almost entirely in the urine, only
about 2 percent of the total dose ultimately appearing in the feces.
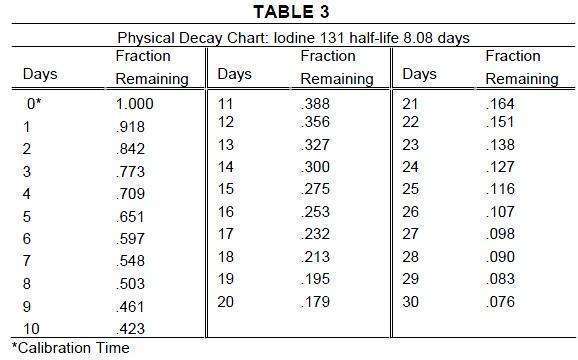
The biologic half-life of iodinated I 131 albumin is dependent upon a number
of factors, and published studies have varied considerably in their reporting of this
figure. It has ranged, in the literature, from below 10 days to over 20 days. One
important factor affecting the biologic half-life is the initial rate of excretion, and
this depends in part on the quality of the iodinated I 131 albumin. With Volumex,
the biologic half-life in normal individuals has been reported to be approximately
14 days.
Volumex (Iodinated I 131 Allbumin injection) is indicated for use in determinations
of total blood and plasma volumes and in protein turnover studies
None known.
A few instances of hyperpyrexia and aseptic (chemical) meningeal irritation have been reported
with the use of iodinated I 131 albumin in cisternography. Iodi-
nated I 131 Albumin Injection is not approved for use in cisternography.
General
In the use of any radioactive material, care should be taken to insure minimum
radiation exposure to the patient and occupational workers consistent with proper
patient management.
Radiopharmaceuticals should be used only by physicians who are qualified by
training and experience in the safe use and handling of radionuclides and whose
experience and training have been approved by the appropriate government
agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic po-
tential or whether iodinated I 131 albumin affects fertility in males or females.
Pregnancy Category C
Animal reproduction studies have not been conducted with Iodinated I 131
Albumin Injection. It is also not known whether this agent can cause fetal harm
when administered to a pregnant woman or can affect reproduction capacity.
Iodinated I 131 Albumin Injection should be administered to a pregnant woman
only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in
nature, of a woman of childbearing capability should be performed during the first
few (approximately 10) days following the onset of menses.
Nursing Mothers
Since iodine 131 is excreted in human milk during lactation, formula feedings
should be substituted for breast feedings.
Pediatric use
Safety and effectiveness in children have not been established.
Although the immunological properties of albumin human are believed to be
virtually unaltered by the iodination process, there is a theoretical possibility that
allergic reactions may occur in patients receiving additional doses a number of
weeks after an initial dose.
Volumex (Iodinated I 131 Albumin Injection) is administered intravenously.
Parenteral drug products should be inspected visually for particulate matter and
abnormal coloration prior to administration whenever solution and container per-
mit.
Volumex (Iodinated I 131 Albumin Injection) may be colorless to very pale
yellow. Solutions with excessive coloration should not be used.
When a procedure such as a blood volume or a circulation time determination
is to be repeated, the total dosage administered in any one week should not
exceed 200 microcuries.
To minimize the uptake of radioactive iodine by the thyroid, prior administration
of Lugol’s Solution (Strong Iodine Solution USP) may be used. Ten drops of
Lugol’s Solution three times daily, beginning at least 24 hours before administra-
tion of Volumex and continuing for one or two weeks thereafter, is a suitable
dose.
Complete assay data for each single unit dose are provided on the container.
Note: The expiration date given on the container pertains to the biologic
properties of the material and not to the radioactivity level. It is important to make
certain that the radioactivity in the dose at the time of administration is sufficient
for the intended use.
The patient dose should be measured by a suitable radioactivity calibration
system immediately prior to administration.
Note: A shielded syringe may be used for withdrawing and injecting the
iodinated I 131 albumin.
Total Blood and Plasma Volumes
Dosage may range from 5 to 50 microcuries.
Blood Volume Determination
A. Reference Solution
Reference solution is provided with each single unit dose of Volumex. Determine
the radioactivity concentration (net cpm/mL) of the reference solution. Care must
be taken to assure that the reference solution and the blood samples (Step B3)
are assayed using the same geometric configuration.
B. Administration of Dose
1. After withdrawing a background blood sample as described below, insert an
intravenous (IV) line into a large vein in patient’s arm. Inject the entire
Volumex dose (one milliliter) through the IV line for immediate delivery.
Measure the residual radioactivity in the syringe and needle.
2. Destroy syringe after injecting. Do not attempt to resterilize. CAUTION: The
syringe should be disposed of in accordance with the US Nuclear Regulatory
Commission or Agreement State regulations pertaining to the disposal of ra-
dioactive waste.
3. At 12, 18, 24, 30 and 36 minutes after injecting the dose, withdraw blood
samples from the patient with a sterile syringe or evacuated
sample tube containing anti-coagulant.
C. Calculation of Blood Volume
1. Take a known aliquot from each blood sample and determine radioconcentration
in net cpm/mL.
2. Plot the 12, 18, 24, 30 and 36 minute sample counts (net cpm/mL) on semilog-
graph paper using the average count value of each sample and determine the
radioconcentration at injection time (zero time) by drawing a straight line through
the 12, 18, 24, 30 and 36 minute points to zero time. The x ordinate of the
graph is the sample withdrawal time and the logarithmic y ordinate is
radioconcentration in net cpm/mL.
3. Calculate patient’s blood volume (in mL) using the following formula:
Net cpm/mL reference solution blood
Net cpm/mL patient’s blood sample x DF = volume
(in mL)
Sample Blood Volume Calculations
Volume of Blood sample aliquot = 1.0 mL
Volume of Reference Solution aliquot = 1.0 mL
Net counts at zero time = 48,100
Net counts obtained from reference solution aliquot = 52, 430
If, for example, DF, dilution factor of the reference solution = 4000
Using the above formula gives = 52,430 x 4000 = 4360 mL
48,100
Serial Blood Volume Determinations
Iodinated I 131 Albumin Injection is administered in sufficiently low dosage to permit
repetitions as often as required by clinical circumstances. It must be remembered
that it is always necessary to correct for background radioactivity remaining in the
blood from former determinations. Therefore, for each determination after the first
one, a background blood sample must be taken just before the iodinated I 131
albumin is injected.
Background Blood Sample
1. Withdraw background blood sample from large vein in patient’s sampling arm
with a sterile syringe or evacuated sampling tube containing anti-coagulant.
2. Leaving needle in patient’s vein, detach syringe containing blood sample.
3. Attached syringe containing the dose of Volumex to the indwelling IV line
and administer (see instructions under Blood Volume Determination,
Administration of Dose).
4. Determine radioconcentration in net cpm/mL of aliquots taken from back-
ground and postinjection blood samples, and from the reference solution.
The radioconcentration (net cpm/mL) per aliquot of the background blood
sample must be subtracted from the radioconcentration per aliquot of the blood
sample obtained after the injection of iodinated I 131 albumin. The formula
for calculating each blood volume determination after the first one thus becomes:
Net cpm/mL reference solution blood x DF = blood
Net cpm/mL Net cpm/mL volume
postinjection minus background (in mL)
blood sample blood sample
Plasma Volume Determination
The procedure is essentially the same as that for blood volume determination,
except that the blood sample drawn from the patient is centrifuged, the red blood
cells are removed, and net cpm/mL of the plasma is determined. The formula for
calculation of plasma volume, therefore is:
Net cpm/mL reference solution x DF = plasma
Net cpm/mL patient’s plasma sample volume
(in mL)
Protein Turnover Studies
Dosages used have ranged from 10 to 150 microcuries. After injection, a period
of seven days should be allowed before determinations are made to permit the
elimination of any degraded protein in the dose.
Radiation Dosimetry
The estimated absorbed radiation doses to an average patient (70 kg) from an
intravenous injection of 50 microcuries of Iodinated I 131 Albumin Injection USP
are shown in Table 4.
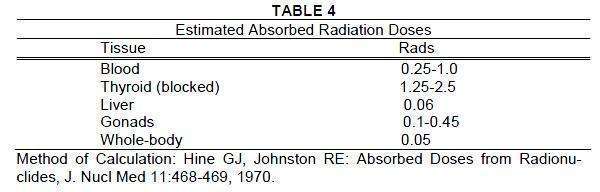
For doses of 10, 25, 75, 150, 500 and 750 microcuries, the estimated absorbed
doses are 0.2, 0.5, 1.5, 3, 10 and 15 times the number of rads given, respectively.
Volumex (Iodinated I 131 Albumin Injection USP) is available in single unit dose
syringes containing 25 microcuries of activity in one milliliter per syringe on
the date of calibration. Each single unit dose syringe is supplied with reference
solution. Complete assay data for each single unit dose syringe and reference
solution are provided on their respective containers.
The maximum concentration of a single unit dose syringe of Volumex does not
exceed 26.3 microcuries per milliliter at time of calibration
Store in a refrigerator, 2-8°C (36-46°F)
This radiopharmaceutical is licensed by the Texas Department of Health, Bureau of Radiation Control for distribution to persons licensed
pursuant to 41.26 (b) and Appendix 41-C, Group I and Group II, "Texas Regulations for Control of Radiation," or under equivalent licenses of the
U.S. Nuclear Regulatory Commission, an Agreement State, or a Licensing State.
Manufactured for Daxor Corporation
350 Fifth Avenue, Suite 7120 · New York, NY 10118 · 212-244-0804
by Iso-Tex Diagnostics, Inc., Friendswood, Texas 77546

VolumexIodinated I-131 Albumin INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||