VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2
222614
FULL PRESCRIBING INFORMATION: CONTENTS*
- VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2 DESCRIPTION
- VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2 INDICATIONS AND USAGE
- WARNINGS
- OTC ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2 DOSAGE AND ADMINISTRATION
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- OTC - WHEN USING
- DO NOT USE
- STOP USE
FULL PRESCRIBING INFORMATION
VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2 DESCRIPTION
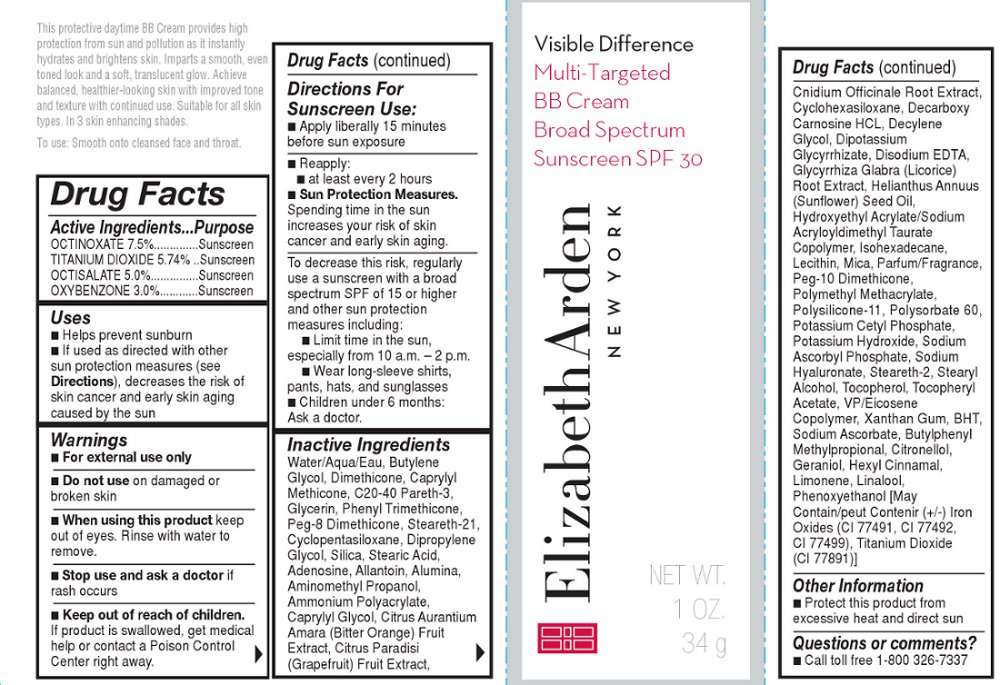
This protective daytime BB Cream provides high protection from sun and pollution as it instantly hydrates and brightens skin. Imparts a smooth, even toned look and a soft, translucent glow.
VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2 INDICATIONS AND USAGE
Smooth onto cleansed face and throat.
WARNINGS
For external use onlyOTC ACTIVE INGREDIENT
OCTINOXATE 7.5%..............Sunscreen
TITANIUM DIOXIDE 5.74% ..Sunscreen
OCTISALATE 5.0%...............Sunscreen
OXYBENZONE 3.0%............Sunscreen
INACTIVE INGREDIENT
Water/Aqua/Eau, Butylene Glycol, Dimethicone, Caprylyl Methicone, C20-40 Pareth-3, Glycerin, Phenyl Trimethicone, Peg-8 Dimethicone, Steareth-21, Cyclopentasiloxane, Dipropylene Glycol, Silica, Stearic Acid, Adenosine, Allantoin, Alumina, Aminomethyl Propanol, Ammonium Polyacrylate, Caprylyl Glycol, Citrus Aurantium Amara (Bitter Orange) Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cnidium Officinale Root Extract, Cyclohexasiloxane, Decarboxy Carnosine HCL, Decylene Glycol, Dipotassium Glycyrrhizate, Disodium EDTA, Glycyrrhiza Glabra (Licorice) Root Extract, Helianthus Annuus (Sunflower) Seed Oil, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Lecithin, Mica, Parfum/Fragrance, Peg-10 Dimethicone, Polymethyl Methacrylate, Polysilicone-11, Polysorbate 60, Potassium Cetyl Phosphate, Potassium Hydroxide, Sodium Ascorbyl Phosphate, Sodium Hyaluronate, Steareth-2, Stearyl Alcohol, Tocopherol, Tocopheryl Acetate, VP/Eicosene Copolymer, Xanthan Gum, BHT, Sodium Ascorbate, Butylphenyl Methylpropional, Citronellol, Geraniol, Hexyl Cinnamal, Limonene, Linalool, Phenoxyethanol [May Contain/peut Contenir (+/-) Iron Oxides (CI 77491, CI 77492, CI 77499), Titanium Dioxide (CI 77891)]
VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2 DOSAGE AND ADMINISTRATION
Smooth onto face and throat
OTC - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.OTC - PURPOSE
Provides SPF 30 sun protection
OTC - WHEN USING
Keep out of eyes. Rinse with water to remove
DO NOT USE
Do not useon damaged or broken skin
STOP USE
If rash occurs
VISIBLE DIFFERENCE MULTI TARGETED BB CREAM BROAD SPECTRUM SUNSCREEN SPF 30 SHADE 2OCTINOXATE, TITANIUM DIOXIDE, OCTISALATE, and OXYBENZONE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||