Virdec DM Drops
Virtus Pharmaceuticals, LLC
Virtus Pharmacueticals, LLC
Virdec DM Drops
FULL PRESCRIBING INFORMATION
Drug Facts
Phenylephrine HCl 3.5 mg
Chlorpheniramine Maleate 1 mg
Dextromethorphan HBr 3 mg
Nasal Decongestant
Antihistamine
Cough Suppressant
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- cough due to minor throat and bronchial irritation
- nasal congestion
- reduces swelling of nasal passages
Do not exceed recommended dosage.
Do not use this product in a child who is
- now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- a persistent or chronic cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- sedatives and tranquilizers may increase drowsiness effect
- nervousness, dizziness, or sleeplessness occur
- cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. These could be signs of a serious condition.
- new symptoms occur
In case of accidental overdose, seek professional help or contact a Poison Control Center immediately.
Directions
Do not exceed recommended dosage.
| Children 6 to under 12 years of age: | 1 dropperful (1 mL) every 4 to 6 hours, not to exceed 6 dropperfuls in 24 hours or as directed by a doctor |
| Children under 6 years of age: | Consult a doctor |
Other information
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
Citric Acid, FD&C Blue # 1, FD&C Red # 40, Glycerin, Grape flavor, Methyl Paraben, Potassium Citrate, Potassium Sorbate, Propyl Paraben, Propylene Glycol, Purified Water, Sucralose
Question? Comments?
Call 813-283-1344
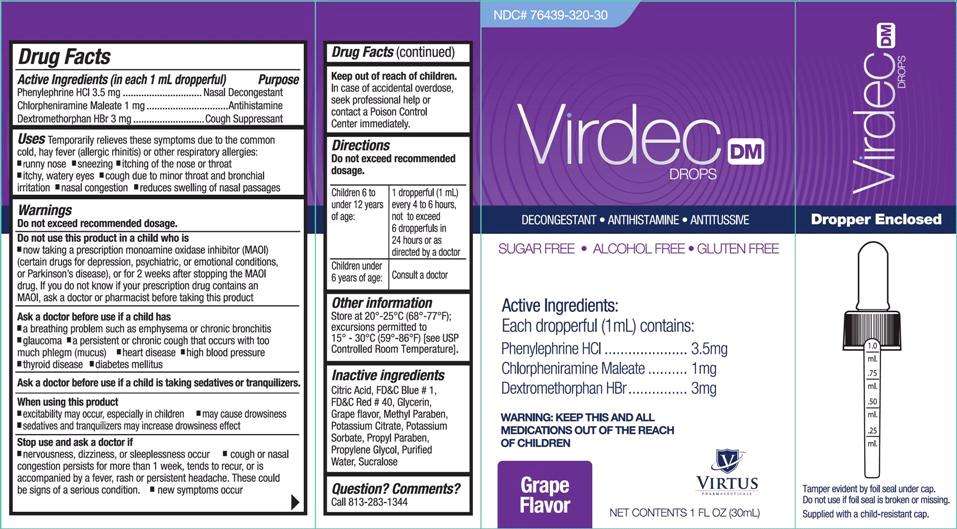
NDC 76439-320-30
Virdec DM Drops
Decongestant • Antihistamine • Antitussive
Sugar Free • Alcohol Free • Gluten Free
Active Ingredient:
Each dropperful (1mL) contains:
Phenylephrine HCl……………….3.5 mg
Chlorpheniramine Maleate………1 mg
Dextromethorphan HBr………….3 mg
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN
Grape Flavor
Virtus Pharmceuticals
NET CONTENTS 1 FL OZ (30 mL)
Dropper Enclosed
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
Supplied with a child resistant cap.
Virdec DM DropsVirdec DM Drops LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||