Vicks
Procter & Gamble Manufacturing GmbH
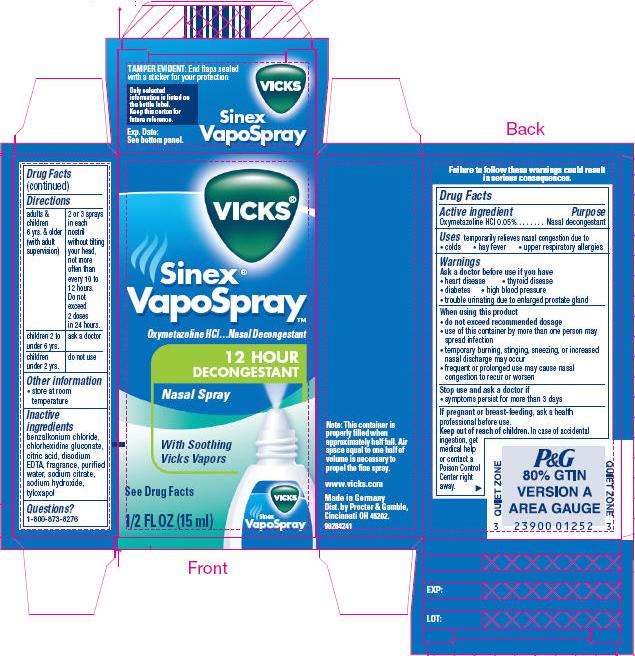
VICKS Sinex VapoSpray 12 HOUR DECONGESTANT Nasal Spray
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Vicks Uses
- Warnings
- Directions
- Vicks Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 15 ml nasal spray container
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Oxymetazoline HCl 0.05%
Purpose
Nasal decongestant
Vicks Uses
temporarily relieves nasal congestion due to
- colds
- hay fever
- upper respiratory allergies
Warnings
Ask a doctor before use if you have
- heart disease
- thyroid disease
- diabetes
- high blood pressure
- trouble urinating due to enlarged prostate gland
When using this product
- do not exceed recommended dosage
- use of this container by more than one person may spread infection
- temporary burning, stinging, sneezing, or increased nasal discharge may occur
- frequent or prolonged use may cause nasal congestion to recur or worsen
Stop use and ask a doctor if
- symptoms persist for more than 3 days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Directions
| adults & children 6 yrs. & older (with adult supervision) | 2 or 3 sprays in each nostril, without tilting your head, not more often than every 10 to 12 hours. Do not exceed 2 doses in 24 hours. |
| children 2 to under 6 yrs. | ask a doctor |
| children under 2 yrs. | do not use |
Vicks Other information
- store at room temperature
Inactive ingredients
benzalkonium chloride, chlorhexidine gluconate, citric acid, disodium EDTA, fragrance, purified water, sodium citrate, sodium hydroxide, tyloxapol
Questions?
1-800-873-8276
www.vicks.com
Made in Germany
Dist. by Procter & Gamble,
Cincinnati OH 45202.
PRINCIPAL DISPLAY PANEL - 15 ml nasal spray container
VICKS®
Sinex®
VapoSpray™
Oxymetazoline HCl…Nasal Decongestant
12 HOUR
DECONGESTANT
Nasal Spray
With Soothing
Vicks Vapors
See Drug Facts
1/2 FL OZ (15 ml)
VicksOXYMETAZOLINE HYDROCHLORIDE SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||