Vanilla Hand Sanitizer
Vanilla Hand Sanitizer
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Active Ingredient
- Purpose
- Use
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Package Label
FULL PRESCRIBING INFORMATION
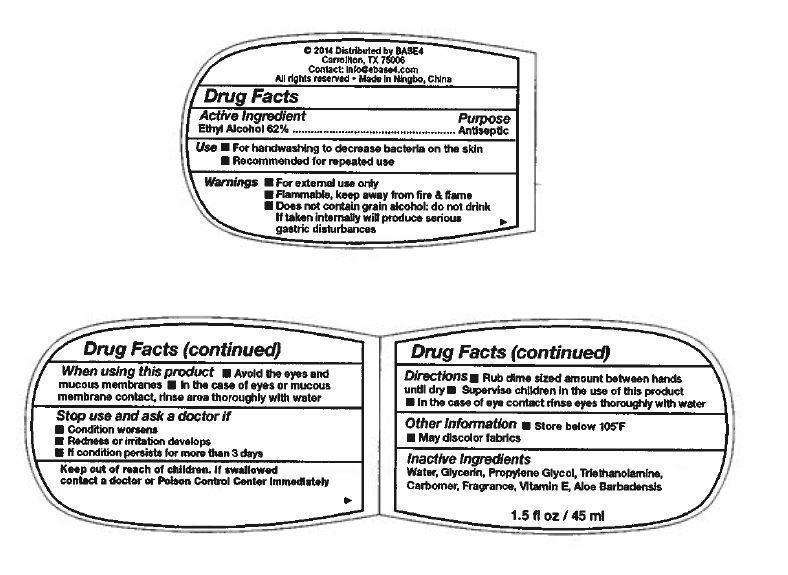
Drug Facts
Active Ingredient
Ethyl Alcohol 62%
Purpose
Antiseptic
Use
- For handwashing to decrease bacteria on skin
- Recommended for repeated use
Warnings
- For external use only
- Flammable, keep away from fire & flame
- Does not contain grain alcohol; do not drink
If taken internally will produce serious gastric distrubances
When using this product
- Avoid the eyes and mucous membrances
- In the case of eyes or mucous membrance contact, rinse area thoroughly with water
Stop use and ask a doctor if
- Condition worsens
- Redness or irritation develops
- If condition persists for more than 3 days
Keep out of reach of children.
If swallowed contact a doctor or Poison Control Center immediately
Directions
- Rub dime sized amount between hands until dry
- Supervise children in the use of this product
- in the case of eye contact rinse eyes thoroughly with water
Other Information
- Store below 105°F
- May discolor fabrics
Inactive Ingredients
Water, Glycerin, Propylene Glycol, Triethanolamine, Carbomer, Fragrance, Vitamin E, Aloe Barbadensis
Package Label
VANILLA
hand sanitizer
© 2014 Distributed by BASE4
Carrollton, TX 75006
Contact: info@base4.com
All rights reserved - Made in Ningbo, China
1.5 fl oz / 45 ml

Vanilla Hand SanitizerALCOHOL GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!