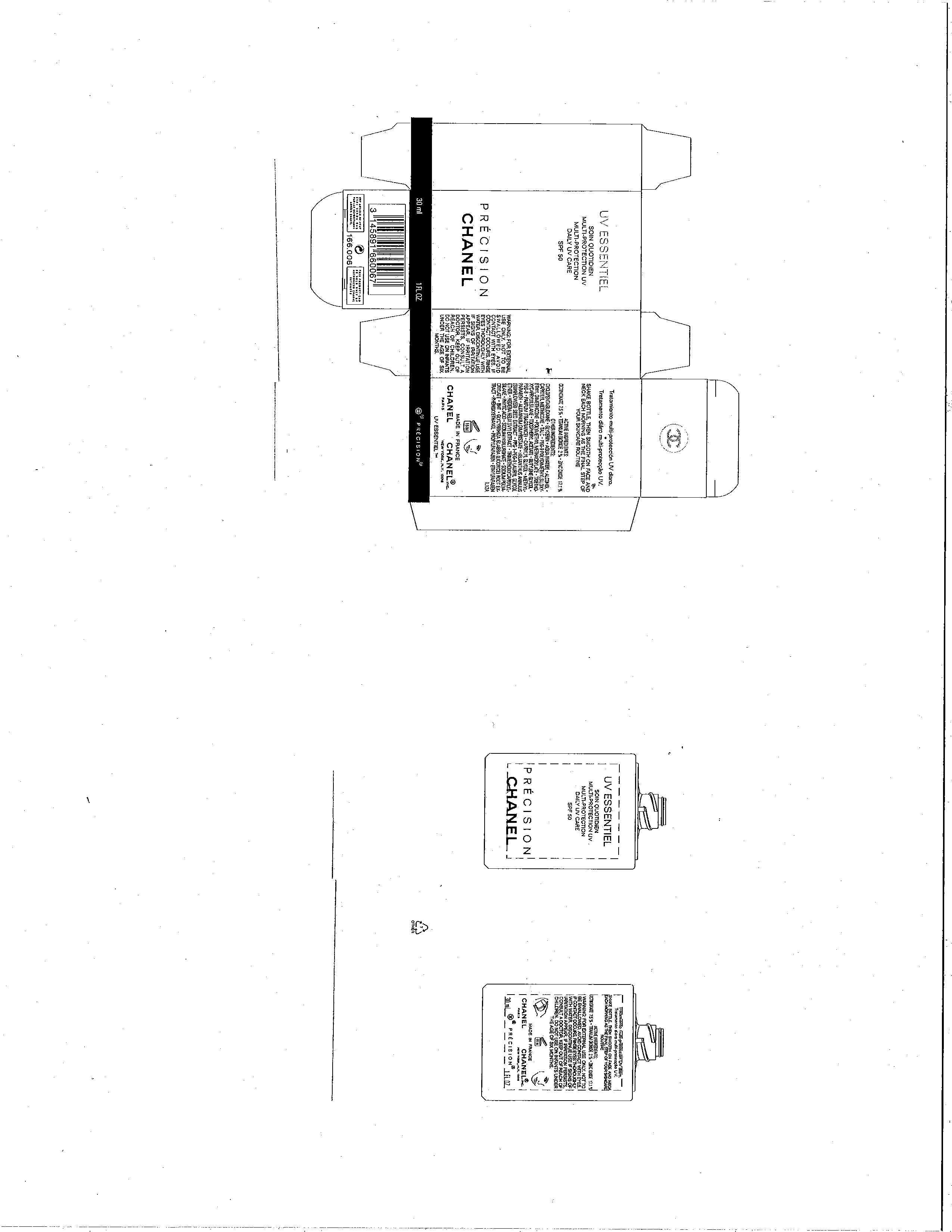
UV ESSENTIEL
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT(S)
Octinoxate 7.5%
Titanium Dioxide 2%
Zinc Oxide 17.1%
WARNING
FOR EXTERNAL USE ONLY. NOT TO BE SWALLOWED. AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER, DISCONTINUE USE IF SIGNS OF IRRITATION APPEAR. IF IRRITATION PERSISTS, CONSULT A DOCTOR. KEEP OUT OF REACH OF CHILDREN. DO NOT USE ON INFANTS UNDER THE AGE OF SIX MONTHS.
DIRECTIONS FOR USE
SHAKE BOTTLE. THEN SMOOTH ON FACE AND NECK EACH MORNING AS THE FINAL STEP OF YOUR SKINCARE ROUTINE.
INACTIVE INGREDIENT(S)
CYCLOPENTASILOXANE, GLYCERIN, AQUA (WATER), ALCOHOL, CAPRYLYL METHICONE, TALC, PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, POLYMETHYL METHACRYLATE, TRIETHOXYCAPRYLYLSILANE, TOCOPHERYL ACETATE, BUTYLENE GLYCOL, PEG-8, PARFUM (FRAGRANCE), CAPRYLYL GLYCOL, METHYLPARABEN, ALUMINUM DIMYRISTATE, HELIANTHUS ANNUUS (SUNFLOWER) SEED EXTRACT, PPG-1-PEG-9 LAURYL GLYCOL ETHER, HEDERA HELIX (IVY) EXTRACT, TRIMETHOXYCAPRYLYLSILANE, PHYTIC ACID, SODIUM HYALURONATE, SODIUM POLYACRYLATE, BHT, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, PHENOXYETHANOL, PROPYLPARABEN, ETHYLPARABEN
PRINCIPAL DISPLAY PANEL
UV Essentiel
Multi-Protection
Daily UV Care
SPF 50
Precision
Chanel
30 ml 1 FL.OZ.
UV ESSENTIELMulti-Protection Daily UV Care EMULSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||