Tyzine Pediatric
PharmaDerm A division of Fougera Pharmaceuticals Inc.
TYZINE NASAL SOLUTION
FULL PRESCRIBING INFORMATION: CONTENTS*
- TYZINE PEDIATRIC DESCRIPTION
- CLINICAL PHARMACOLOGY
- TYZINE PEDIATRIC INDICATIONS AND USAGE
- TYZINE PEDIATRIC CONTRAINDICATIONS
- WARNINGS
- TYZINE PEDIATRIC ADVERSE REACTIONS
- OVERDOSAGE
- TYZINE PEDIATRIC DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Container
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Carton
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Container
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Carton
FULL PRESCRIBING INFORMATION
Rx Only
TYZINE PEDIATRIC DESCRIPTION
Tyzine® Nasal Solution contains tetrahydrozoline hydrochloride, 2-(1,2,3,4-Tetrahydro-1, naphthyl)-2-imidazoline monohydrochloride, as a nasal decongestant. The chemical structure is:
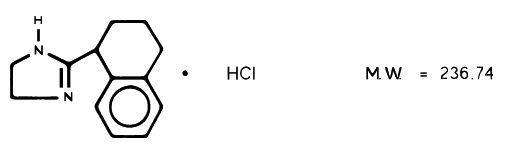
Nasal Solution is available for topical nasal application as 0.1% nasal solution and as 0.05% pediatric nasal drops. The nasal solution is a solution of tetrahydrozoline hydrochloride in water, with sodium chloride, sodium citrate, edetate disodium, and benzalkonium chloride, with hydrochloric acid to adjust to the pH.
CLINICAL PHARMACOLOGY
Tyzine® Nasal Solution (tetrahydrozoline hydrochloride), a sympathomimetic amine, possesses vasoconstrictor and decongestant actions when applied to nasal mucosa, resulting in vasoconstriction of the smaller arterioles of the nasal passages. Information on the absorption, distribution and elimination of the drug is not available.
TYZINE PEDIATRIC INDICATIONS AND USAGE
Tyzine® Nasal Solution is indicated for decongestion of nasal and nasopharyngeal mucosa.
TYZINE PEDIATRIC CONTRAINDICATIONS
Tyzine® Nasal Solution is contraindicated for patients who have shown previous hypersensitivity to its components. The 0.1% solution is contraindicated in children under six years of age. Tyzine® Nasal Solution is not to be used for infants under two years of age. Tyzine® Pediatric Nasal Drops (0.05%) should be used for children between the ages of 2 and 6 years (See "Dosage and Administration"). Tyzine® Nasal Solution should not be used by patients under treatment with Monoamine Oxidase (MAO) Inhibitors.
WARNINGS
Overdose in children may produce profound sedation. This may be accompanied by profuse sweating, hypotension or even shock (See "Overdosage").
General
Avoid doses greater or more frequent than those recommended below. Excessive dosage in children may, on rare occasions, cause severe drowsiness. Profuse sweating may accompany this effect. Overdosage may also cause marked hypotension or even shock. Use cautiously in patients with cardiovascular disease (e.g., coronary artery disease, hypertension), and metabolic-endocrine diseases (e.g., hyperthyroidism, diabetes).
Information for Patients
Patients should be advised to follow the prescribed dosage regimen. The spray should be administered with the head held upright. To spray, squeeze bottle quickly and firmly and sniff briskly. Instillation of the nose drops can be most conveniently accomplished with the patient in the lateral head-low position.
Drug Interactions
| DRUG | EFFECT |
|---|---|
| Monoamine Oxidase (MAO) Inhibitors |
Hypertension |
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Tyzine® . It is also not known whether Tyzine® Nasal Solution can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tyzine® Nasal Solution should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when Tyzine® Nasal Solution is administered to a nursing woman.
TYZINE PEDIATRIC ADVERSE REACTIONS
Local application of Tyzine® Nasal Solution can be associated with burning, stinging, sneezing or dryness of the mucosa. Occasionally, systemic sympathomimetic effects can occur, including headaches, drowsiness, weakness, tremors, light-headedness, insomnia, and palpitations. Rebound congestion can also occur, and is characterized by chronic swelling of the nasal mucosa resulting in chronic redness, swelling and rhinitis. If adverse reactions occur, discontinue use.
OVERDOSAGE
The administration or ingestion of overdoses of Tyzine® (tetrahydrozoline hydrochloride) Nasal Solution may result in oversedation in young children. Overdoses have caused hypertension, bradycardia, drowsiness and rebound hypotension in adults; a shock-like syndrome with hypotension and bradycardia may also occur. In either case, the treatment of overdosage is usually that of watchful expectancy and general supportive measures. The patient should be kept warm, fluid balance should be maintained orally, if possible, and parenterally, if necessary.
If the respiratory rate drops to 10 or below, the patient should be given oxygen, and respiration assisted. Blood pressure should be watched carefully to prevent a hypotensive crisis.
There is no known antidote for Tyzine® Nasal Solution. The use of stimulants is contraindicated. To date, we have had no report of fatalities resulting from overdosages of Tyzine® Nasal Solution and while the symptoms resulting from Tyzine® Nasal Solution overdosage may be alarming, they are self-limiting and the patient recovers with no sequelae.
TYZINE PEDIATRIC DOSAGE AND ADMINISTRATION
Adults and Children 6 Years and Over
Tyzine 0.1% Nasal Solution
It is recommended that 2 to 4 drops of Tyzine® 0.1% Nasal Solution be instilled in each nostril as needed, never more often than every three hours. Less frequent administration is usually sufficient since relief is maintained for four hours or longer in most cases, and often for as long as eight hours. Bedtime instillation usually assures sleep undisturbed by the need for remedication before morning, or by insomnia from central stimulation.
Tyzine 0.1% Nasal Spray
It is recommended to squeeze quickly and firmly three or four times Tyzine® 0.1% Nasal Spray in each nostril as needed, never more often than every three hours. Less frequent administration is usually sufficient since relief is maintained for four hours or longer in most cases, and often for as long as eight hours. Bedtime instillation usually assures sleep undisturbed by the need for remedication before morning, or by insomnia from central stimulation.
Children 2 to 6 Years of Age
Tyzine 0.05 % Pediatric Nasal Drops
Note: Do not use Tyzine® 0.1% Nasal Solution or Tyzine® 0.1% Nasal Spray
It is recommended that 2 to 3 drops of Tyzine® 0.05% Pediatric Nasal Drops be instilled in each nostril as needed, and never more often than every three hours. Relief usually lasts for several hours, so that instillation is usually needed only every four to six hours. Instillation of nose drops can be most conveniently accomplished with the patient in the lateral head-low position.
HOW SUPPLIED
Tyzine® Nasal Solution (0.1%) is available in:
30 mL bottles - NDC 10337-476-30
15 mL Nasal Spray Bottles- NDC 10337-476-15
Tyzine® Pediatric Nasal Drops (0.05%) is available in:
15 mL bottles - NDC 10337-477-15
Recommended Storage
Store below 86°F (30°C).
Rx Only
Manufactured for:
PharmaDerm®
A division of Nycomed US Inc.
Melville, NY 11747 USA
www.pharmaderm.com
By Denison Pharmaceuticals, Inc.
Pawtucket, Rhode Island 02862
©Copyright 2000 Kenwood Therapeutics
IL062A
Rev. 06/11
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Container
NDC 10337-477-15
Rx Only
PEDIATRIC NASAL DROPS
TYZINE®
(tetrahydrozoline hydrochloride)
0.05% SOLUTION
15 mL (1/2 FL. OZ.)
PharmaDerm®
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Carton
NDC 10337-477-15
Rx Only
PEDIATRIC NASAL DROPS
TYZINE®
(tetrahydrozoline hydrochloride)
0.05% SOLUTION
15 mL (1/2 FL. OZ.)
PharmaDerm®
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Container
NDC 10337-476-15
Rx Only
NASAL SPRAY
TYZINE®
(tetrahydrozoline hydrochloride)
0.1% SOLUTION
15 mL (1/2 FL. OZ.)
PharmaDerm®
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15mL Carton
NDC 10337-476-15
Rx Only
NASAL SPRAY
TYZINE®
(tetrahydrozoline hydrochloride)
0.1% SOLUTION
15 mL (1/2 FL. OZ.)
PharmaDerm®
Tyzine PediatricTETRAHYDROZOLINE HYDROCHLORIDE SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TyzineTETRAHYDROZOLINE HYDROCHLORIDE SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||