Tylosin Tartrate
AMERICAN PHARMACEUTICAL INGREDIENTS LLC
Tylosin Tartrate
FULL PRESCRIBING INFORMATION
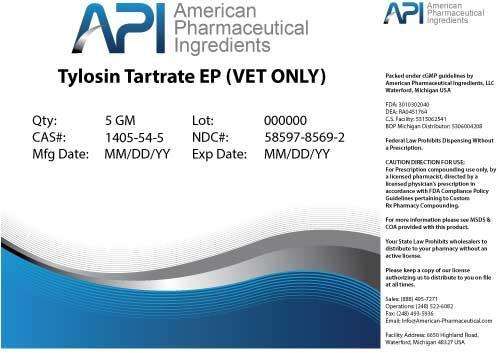
Tylosin Tartrate
Tylosin Tartrate POWDER
Product Information
|
|
Product Type
|
Bulk ingredient |
Item Code (Source)
|
NDC:58597-8569 |
|
Route of Administration
|
NOT APPLICABLE |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
tylosin tartrate tylosin |
|
1 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:58597-8569-2 |
5 in 1 BOTTLE |
|
|
|
2 |
NDC:58597-8569-4 |
25 in 1 BOTTLE |
|
|
|
3 |
NDC:58597-8569-6 |
100 in 1 BOTTLE |
|
|
|
4 |
NDC:58597-8569-7 |
500 in 1 BOTTLE |
|
|
|
5 |
NDC:58597-8569-8 |
1000 in 1 BOTTLE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2014-05-02 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!
