Trioxin
TriOxin OTIC SUSPENSION
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRIOXIN DESCRIPTION
- TRIOXIN INDICATIONS AND USAGE
- TRIOXIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TRIOXIN ADVERSE REACTIONS
- OVERDOSE
- TRIOXIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL-Sample Label
- PRINCIPAL DISPLAY PANEL-Sample Carton
- PRINCIPAL DISPLAY PANEL-15 mL Carton
FULL PRESCRIBING INFORMATION
Rx Only
TRIOXIN DESCRIPTION
Each 1 mL for otic administration contains:
Chloroxylenol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 mg
Benzocaine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15 mg
Hydrocortisone Acetate . . . . . . . . . . . . . . . . . . . . . . . . . . 10 mg
INACTIVE INGREDIENTS: PEG-12 Glyceryl Dimyristate, Sodium Hydroxide, PEG-40 HCO, Purified Water, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Xanthan Gum, Potassium Sorbate, Isopropyl Alcohol.
CLINICAL PHARMACOLOGY
Sander R. Otitis Externa: A Practical Guide to Treatment and Prevention. Available at http://www.aafp.org/afp/20010301/927.html. Accessed 4/9/09.
BioZone Laboratories, Inc. Qusomes™. Available at: http://www.biozonelabs.com/html/qusomes/qusomes.htm. Accessed 4/15/09.
TriOxin is effective both as an antibacterial and antifungal agent. The unique Hydrophilic Lipid based delivery system provides a suspension having an acid pH and a low surface tension, exerting a drying effect and allowing the medication to spread quickly to all contiguous surfaces, softening and reducing accumulated cerumen. Chloroxylenol is a halogenated phenol; non-toxic, non-corrosive, non-staining with high phenol coefficient. It may be applied directly to a wound and shows no chemical reactivity toward blood. TriOxin which contains hydrocortisone acetate is indicated when otitis is complicated by inflammation or to control itching. Benzocaine is a topical anesthetic with a low index of sensitization and toxicity.
TRIOXIN INDICATIONS AND USAGE
For the treatment of superficial infections of the external auditory canal complicated by inflammation caused by organisms susceptible to the action of the antimicrobial. May also be used to control itching in the auditory canal.
TRIOXIN CONTRAINDICATIONS
Topical steroids are contraindicated in varicella, vaccinia and in patients sensitive to any of the components of this preparation. The preparation is not to be used for ophthalmic use and should not be applied in the external auditory canal of patients with perforated eardrums.
WARNINGS
This preparation is not intended for ophthalmic or oral use. If accidental ingestion occurs, seek professional help. If irritation or sensitization occurs, promptly discontinue use of this preparation and institute other measures.
PRECAUTIONS
General
If a favorable response does not occur promptly, discontinue the use of this preparation until the infection is controlled by other appropriate measures. Although systemic side effects are not common with topical corticosteroids, the possibility of occurrence must be kept in mind, particularly when used for an extended period of time.
USAGE IN PREGNANCY
The safety of topical steroid preparations during pregnancy has not been established. Therefore, they should not be used on pregnant patients.
TRIOXIN ADVERSE REACTIONS
The following adverse reactions with topical corticosteroids have been observed: itching, burning, irritation, dryness, folliculitis, hypertrichosis, acneform eruptions, hypopigmentations, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae and miliaria.
OVERDOSE
Topically applied corticosteroids can be absorbed in sufficient amount to produce systemic effects.
TRIOXIN DOSAGE AND ADMINISTRATION
SHAKE WELL BEFORE USING. The external auditory canal should be thoroughly cleansed and dried with a sterile cotton applicator. For adults, 4 to 5 drops of the suspension should be instilled into the affected ear 3 or 4 times daily. For infants and small children, 3 drops are suggested because of the smaller capacity of the ear canal. The patient should lie with the affected ear upward to instill the drops and this position maintained for 5 minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
HOW SUPPLIED
TriOxin Otic Suspension is supplied in 15mL amber glass bottles with dosage dropper and package insert. NDC 68025-041-15
Rx Only
Storage Conditions
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
05/09
Manufactured for
VERTICAL PHARMACEUTICALS, INC.
Sayreville, NJ 08872
Lipid Base Delivery System
QuSomes™
US Patent No. 6,610,322
US Patent No. 6,958,160
US Patent No. 7,150,883
Other patents may apply
PRINCIPAL DISPLAY PANEL-Sample Label
NDC 68025-041-02
TriOxin
Rx Only
Otic Suspension
(Chloroxylenol 1 mg, Benzocaine 15 mg,
Hydrocortisone Acetate10 mg)
2 mL

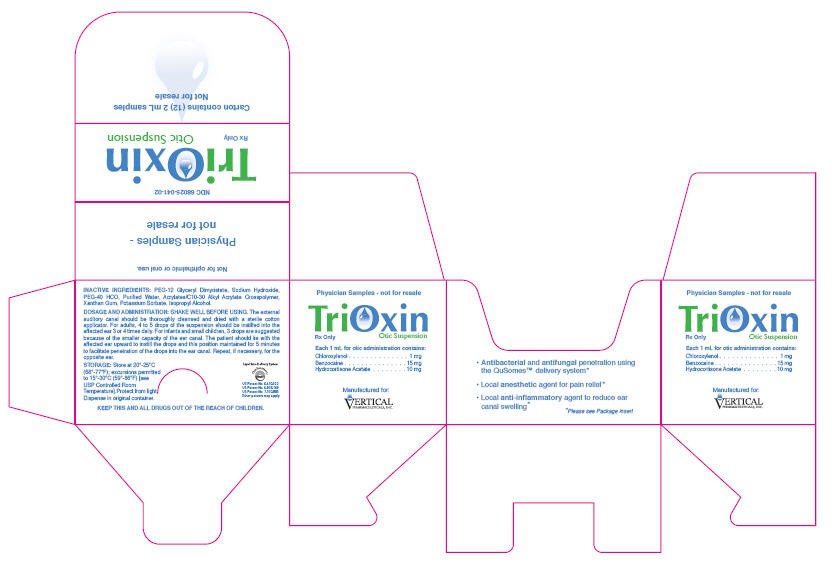
PRINCIPAL DISPLAY PANEL-Sample Carton
Physician Samples - not for resale
TriOxin
Rx Only
Otic Suspension
Each 1 mL for otic administration contains:
Chloroxylenol . . . . . . . . . . . . . . . 1 mg
Benzocaine . . . . . . . . . . . . . . . 15 mg
Hydrocortisone Acetate . . . . . 10 mg
Manufactured for:
VERTICAL
PHARMACEUTICALS, INC.
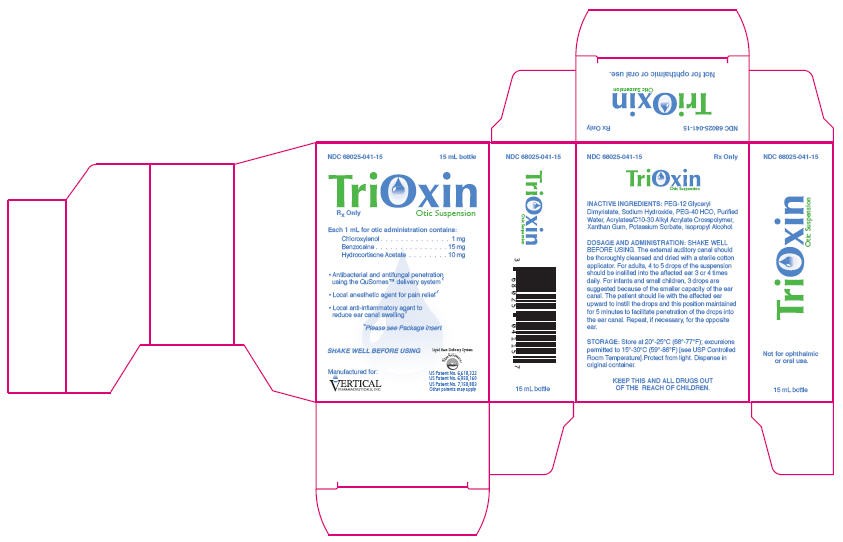
PRINCIPAL DISPLAY PANEL-15 mL Carton
NDC 68025-041-15
15 mL bottle
TriOxin
Rx Only
Otic Suspension
Each 1 mL for otic administration contains:
Chloroxylenol . . . . . . . . . . . . . . . 1 mg
Benzocaine . . . . . . . . . . . . . . . 15 mg
Hydrocortisone Acetate . . . . . 10 mg
• Antibacterial and antifungal penetration
using the QuSomes™ delivery system*
• Local anesthetic agent for pain relief*
• Local anti-inflammatory agent to
reduce ear canal swelling*
*Please see Package insert
SHAKE WELL BEFORE USING
Manufactured for:
VERTICAL
PHARMACEUTICALS, INC.
Lipid Base Delivery System
QuSomes™
US Patent No. 6,610,322
US Patent No. 6,958,160
US Patent No. 7,150,883
Other patents may apply
TrioxinChloroxylenol, Benzocaine and Hydrocortisone Acetate SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||