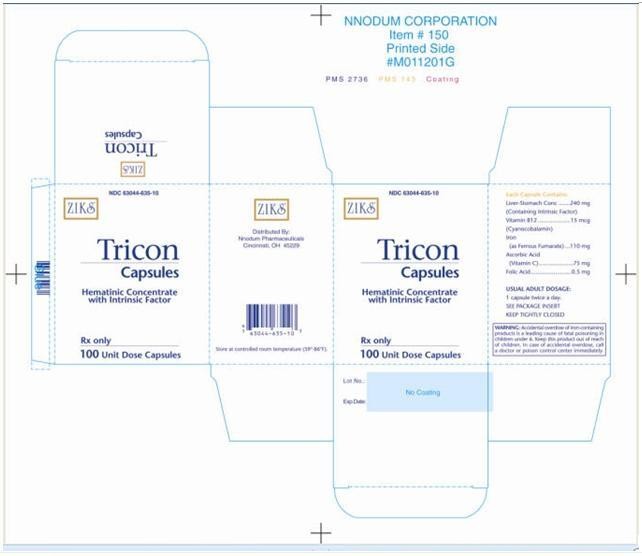
TRICON
TRICON™ Capsules
FULL PRESCRIBING INFORMATION
Liver Stomach Concentrate Capsule
Hematinic Conentrate with Intrinsic Factor
NDC 63044-635-10
Rx Only
| WARNING: Accidental overdose of iron containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. |
A Highly Potent Oral Antianemia Preparation
Each TRICON™ Capsule contains:
Special liver-stomach concentrate
(containing intrinsic factor). . . . . . . . . . . . . . . . 240 mg
Vitamin B12 (activity equivalent) . . . . . . . . . . . .15 mcg
Iron, elemental (ferrous fumarate) . . . . . . . . . .110 mg
Vitamin C (ascorbic acid) . . . . . . . . . . . . . . . . . 75 mg
Folic acid . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.5 mg
with other factors of vitamin B complex present in the liver-stomach concentrate.
Inactive ingredients: Citric acid, D&C Yellow No. 10, ethylcellulose, FD&C Blue No. 1, FD&C Red #28, gelatin, lecithin, magnesium stearate, pharmaceutical glaze, silicon dioxide, simethicone, sodium benzoate, sodium citrate, sorbic acid, starch, and titanium dioxide.
Factor: when secretion of intrinsic factor in gastric juice is inadequate or absent (e.g., Addisonian pernicious anemia or after gastrectomy), vitamin B12 in physiologic doses is absorbed poorly, if at all. The resulting deficiency of vitamin B12 leads to the clinical manifestations of pernicious anemia. Similar megaloblastic anemias may develop in fish tapeworm (Diphyllobothrium latum) infection or after a surgically created small bowel blind loop; in these situations, treatment requires freeing the host of the parasites or bacteria that appear to compete for the available vitamin B12. Strict vegetarianism and malabsorption syndromes may also lead to vitamin B12 deficiency. In the latter case, parenteral therapy or oral therapy with so-called massive doses of vitamin B12 may be necessary for adequate treatment of the patient.
Potency of intrinsic factor concentrates is determined physiologically, i.e., by their use in patients with pernicious anemia. The liver-stomach concetrate with intrinsic factor and the vitamin B12 contained in two TRICON™ Capsules provide 1½ times the minimum amount of therapeutic agent that, when given daily in an uncomplicated case of pernicious anemia, will produce a satisfactory response and relief of anemia and symptoms.
Concentrates of intrinsic factor derived from hog gastric, pyloric, and duodenal mucosa have been used successfully in patients who lack intrinsic factor.
Folic Acid: Folic acid deficiency is the immediate cause of most, if not all, cases of nutritional megaloblastic anemia and of the megaloblastic anemias of pregnancy and infancy; usually, it is also at least partially responsible for the megaloblastic anemias of malabsorption syndromes, e.g., tropical and nontropical sprue.
It is apparent that in vitamin B12 deficiency (e.g., pernicious anemia) lack of this vitamin results in impaired utilization of folic acid. There are other evidences of the close folic acid-vitamin B12 interrelationship: (1) B12 influences the storage, absorption, and utilization of folic acid, and (2) as a deficiency of B12 progresses, the requirement for folic acid increases. However, folic acid does not change the requirements for vitamin B12.
Iron: A very common anemia is that due to iron deficiency. In most cases, the response to iron salts is prompt, safe, and predictable. Within limits, the response is quicker and more certain to large doses of iron than to small doses.
Each TRICON™ (hematinic concentrate with intrinsic factor) Capsule furnishes 110 mg of elemental iron (as ferrous fumarate) to provide a maximum response.
Ascorbic Acid: Vitamin C plays a role in anemia therapy. It augments the conversion of folic acid to its active form, folinic acid. In addition, ascorbic acid promotes the reduction of ferric iron in food to the more readily absorbed ferrous form. Severe and prolonged vitamin C deficiency is associated with an anemia that is usually hypochromic but occasionally megaloblastic in type.
TRICON™ is a multifactor preparation effective in the treatment of anemias that respond to oral hematinics, including pernicious anemia and other megaloblastic anemias and also irondeficiency anemia. Therapeutic quantities of hematopoietic factors that are known to be important are present in the recommended daily dose.
Hemochromatosis and hemosiderosis are contraindications to iron therapy.
Anemia is a manifestation that requires appropriate investigation to determine its cause or causes.
Folic acid alone is unwarranted in the treatment of pure vitamin B12 deficiency states, such as pernicious anemia. Folic acid may obscure pernicious anemia in that the blood picture may revert to normal while neurological manifestations remain progressive.
As with all preparations containing intrinsic factor, resistance may develop in some cases of pernicious anemia to the potentiation of absorption of physiologic doses of vitamin B12. If resistance occurs, parenteral therapy or oral therapy with so-called massive doses of vitamin B12 may be necessary for adequate treatment of the patient. No single regimen fits all cases, and the status of the patient observed in follow-up is the final criterion for adequacy of therapy. Periodic clinical and laboratory studies are considered essential and are recommended.
Pregnancy Category C:
Animal reproduction studies have not been conducted with TRICON™ Capsules. It is also not known whether TRICON™ Capsules can cause fetal harm when administered to pregnant women or can affect reproduction capacity. TRICON™ Capsules should be given to pregnant women only if clearly needed.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TRICON™ is administered to a nursing woman.
Safety and effectiveness in pediatric patients below the age of 10 have not been established.
Geriatric Use: Clinical studies on this product have not been performed in sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
Rarely, iron in therapeutic doses produces gastrointestinal reactions, such as diarrhea or constipation. Reducing the dose and administering it with meals will minimize these effects in the iron-sensitive patient.
In extremely rare instances, skin rash suggesting allergy has been noted following the oral administration of liver-stomach material. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Symptoms: Those of iron intoxication, which may include pallor and cyanosis, vomiting, hematemesis, diarrhea, melena, shock, drowsiness, and coma.
Treatment: For specific therapy, exchange transfusion and chelating agents. For general management, gastric and rectal lavage with sodium bicarbonate solution or milk, administration of intravenous fluids and electrolytes, and use of oxygen.
One capsule twice a day. (Two capsules daily produce a standard response in the average uncomplicated case of pernicious anemia.)
TRICON™ Capsules are opaque brown NDC 63044-0635-10 Unit Dose Packs containing 10 capsules per card. 100 capsules.
Storage: Store at controlled room temperature 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature.
Manufactured For
Nnodum Pharmaceuticals by Contract Pharmacal Corporation
135 Adams Avenue
Hauppauge, New York 11788
TRICONTRICON CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||