Trezix
TREZIX™ Capsules
FULL PRESCRIBING INFORMATION
Acetaminophen, Caffeine and Dihydrocodeine Bitartrate CIII
320.5 mg / 30 mg / 16 mg
Rx Only
*Warning: may be habit-forming.
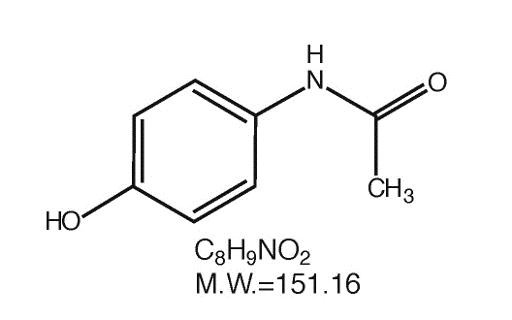
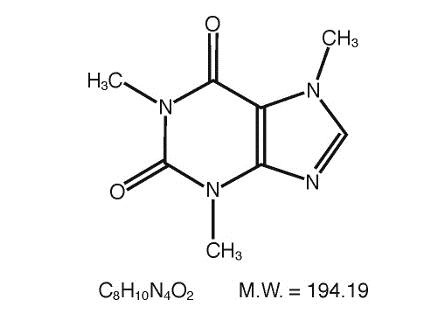
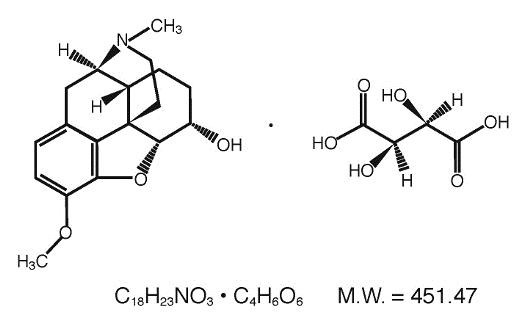
CLINICAL PHARMACOLOGY:
Uses
INDICATIONS AND USAGE:CONTRAINDICATIONS:
WARNINGS:
Hepatotoxicity:
Hypersensitivity/Anaphylaxis:
Usage in Ambulatory Patients:
Respiratory Depression:
Head Injury:
Hypotensive Effect:
Drug Dependence:
DRUG ABUSE AND DEPENDENCE
PRECAUTIONS:
General:
DOSAGE AND ADMINISTRATION
Drug Interactions:
Dihydrocodeine with Other Central Nervous System Depressants:
Dihydrocodeine with Monoamine Oxidase Inhibitors:
Dihydrocodeine with Mixed Agonist/Antagonist Opioid Analgesics:
Acetaminophen Drug Interactions:
Caffeine Drug Interactions:
Information for Patients/Caregivers:
Pregnancy:
Labor and Delivery:
Nursing Mothers:
Pediatric Use:
Geriatric Use:
TREZIX™ capsules should be given with caution to the elderly.
Hepatic Impairment:
TREZIX™ capsules should be given with caution to patients with hepatic insufficiency. Since dihydrocodeine is metabolized by the liver and since acetaminophen potentially causes hepatotoxicity, the effects of this combination product should be monitored closely in such patients.
Renal Impairment:
TREZIX™ capsules should be used with caution and at reduced dosage in the presence of impaired renal function.
ADVERSE REACTIONS:
Dihydrocodeine:
Acetaminophen:
OVERDOSAGE
Caffeine:
DRUG ABUSE AND DEPENDENCE:
OVERDOSAGE:
Signs and Symptoms: dihydrocodeine
acetaminophen caffeine
Treatment
DOSAGE AND ADMINISTRATION:
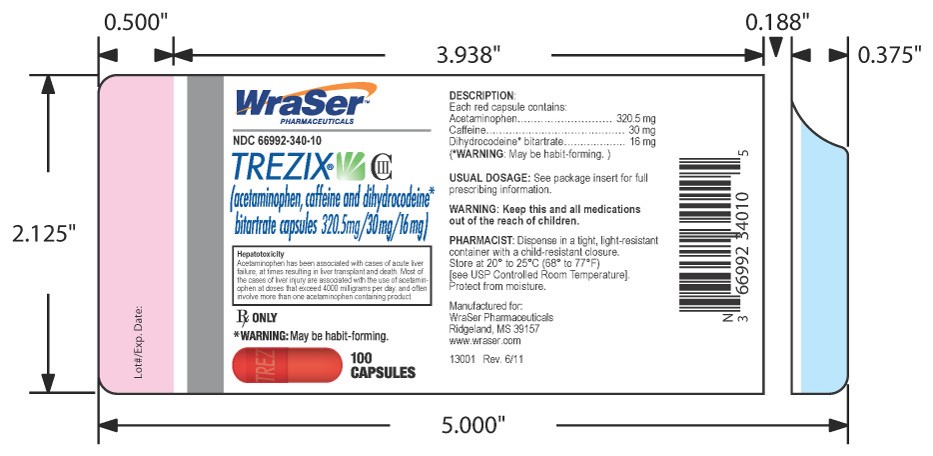
HOW SUPPLIED:
Rx Only
Manufactured for:
WraSer
TM
PHARMACEUTICALS
NDC 66992-340-10
TREZIX® CIII
(acetaminophen, caffeine and dihydrocodeine*
bitartrate capsules 320.5mg/30mg/16mg)
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver
failure, at times resulting in liver transplant and death. Most of
the cases of liver injury are associated with the use of acetaminophen
at doses that exceed 4000 milligrams per day, and often
involve more than one acetaminophen containing product.
Rx ONLY
*WARNING: May be habit-forming.
100 CAPSULES
Trezixacetaminophen, caffeine, dihydrocodeine bitartrate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||