Trazamine
Trazamine
FULL PRESCRIBING INFORMATION
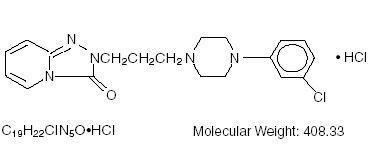
Trazodone - Clinical Pharmacology The mechanism of Trazodone HCl’s antidepressant action in man is not fully understood. In animals, Trazodone HCl selectively inhibits its serotonin uptake by brain synaptosomes and potentiates the behavioral changes induced by the serotonin precursor, 5-hydroxytryptophan. Cardiac conduction effects of Trazodone HCl in the anesthetized dog are qualitatively dissimilar and quantitatively less pronounced than those seen with tricyclic antidepressants. Trazodone HCl is not a monoamine oxidase inhibitor and, unlike amphetamine type drugs, does not stimulate the central nervous system.
Uses
Contraindications Trazodone hydrochloride tablets are contraindicated in patients hypersensitive to Trazodone HCl.
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
|
|
Increases Compared to Placebo |
| Less than 18 | 14 additional cases |
| 18-24 | 5 additional cases |
|
|
Decreases Compared to Placebo |
| 25-64 | 1 fewer case |
| Greater than or equals to 65 | 6 fewer cases |
Pediatric Use Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS—Clinical Worsening and Suicide Risk). Anyone considering the use of Trazodone HCl in a child or adolescent must balance the potential risks with the clinical need.
Adverse Reactions Because the frequency of adverse drug effects is affected by diverse factors (e.g., drug dose, method of detection, physician judgment, disease under treatment, etc.) a single meaningful estimate of adverse event incidence is difficult to obtain. This problem is illustrated by the variation in adverse event incidence observed and reported from the inpatients and outpatients treated with Trazodone HCl.
It is impossible to determine precisely what accounts for the differences observed Clinical Trial Reports Table 2 below is presented solely to indicate the relative frequency of adverse events reported in representative controlled clinical studies conducted to evaluate the safety and efficacy of Trazodone HCl. The figures cited cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice where patient characteristics and other factors often differ from those which prevailed in clinical trials. These incidence figures, also, cannot be compared with those obtained from other clinical studies involving related drug products and placebo as each group of drug trials is conducted under a different set of conditions. Table 2 Treatment Emergent Symptom Incidence InpatientsOutpatients T1 P2 T3 P4 Number of Patients 142 95 157 158 % of Patients Reporting Allergic Skin Condition/Edema 2.8 1.1 7.0 1.3 Autonomic Blurred Vision 6.3 4.2 14.7 3.8 Constipation 7.0 4.2 7.6 5.7 Dry Mouth 14.8 8.4 33.8 20.3 Cardiovascular Hypertension 2.1 1.1 1.3 *5 Hypotension 7.0 1.1 3.8 0.0 Shortness of Breath *6 1.1 1.3 0.0 Syncope 2.8 2.1 4.5 1.3 Tachycardia/Palpitations 0.0 0.0 7.0 7.0 CNS Anger/Hostility 3.5 6.3 1.3 2.5 Confusion 4.9 0.0 5.7 7.6 Decreased Concentration 2.8 2.1 1.3 0.0 Disorientation 2.1 0.0 *7 0.0 Dizziness/Lightheadedness 19.7 5.3 28.0 15.2 Drowsiness 23.9 6.3 40.8 19.6 Excitement 1.4 1.1 5.1 5.7 Fatigue 11.3 4.2 5.7 2.5 Headache 9.9 5.3 19.8 15.8 Insomnia 9.9 10.5 6.4 12.0 Impaired Memory 1.4 0.0 *8 *9 Nervousness 14.8 10.5 6.4 8.2 Gastrointestinal Abdominal/Gastric Disorder Bad Taste in Mouth 1.4 0.0 0.0 0.0 Diarrhea 0.0 1.1 4.5 1.9 Nausea/Vomiting 9.9 1.1 12.7 9.5 Musculoskeletal Musculoskeletal Aches/Pains 5.6 3.2 5.1 2.5 Neurological Incoordination 4.9 0.0 1.9 0.0 Paresthesia 1.4 0.0 0.0 *10 Tremors 2.8 1.1 5.1 3.8 Sexual Function Decreased Libido *11 1.1 1.3 *12 Other Decreased Appetite 3.5 5.3 0.0 *13 Eyes Red/Tired/Itching 2.8 0.0 0.0 0.0 Head Full-Heavy 2.8 0.0 0.0 0.0 Malaise 2.8 0.0 0.0 0.0 Nasal/Sinus Congestion 2.8 0.0 5.7 3.2 Nightmares/Vivid Dreams *14 1.1 5.1 5.7 Sweating/Clamminess 1.4 1.1 *15 *16 Tinnitus 1.4 0.0 0.0 *17 Weight Gain 1.4 0.0 4.5 1.9 Weight Loss *18 3.2 5.7 2.5 1 T=Trazodone HCl 2 P=Placebo 3 4 5 Incidence less than 1% 6 7 8 9 10 11 12 13 14 15 16 17 18 Occasional sinus bradycardia has occurred in long-term studies.
In addition to the relatively common (i.e., greater than 1%) untoward events enumerated above, the following adverse events have been reported to occur in association with the use of Trazodone HCl in the controlled clinical studies: akathisia, allergic reaction, anemia, chest pain, delayed urine flow, early menses, flatulence, hallucinations/delusions, hematuria, hypersalivation, hypomania, impaired speech, impotence, increased appetite, increased libido, increased urinary frequency, missed periods, muscle twitches, numbness, and retrograde ejaculation. Post Introduction Reports: Although the following adverse reactions have been reported in Trazodone HCl users, the causal association has neither been confirmed nor refuted. Voluntary reports received since market introduction include the following: abnormal dreams, agitation, alopecia, anxiety, aphasia, apnea, ataxia, breast enlargement or engorgement, cardiospasm, cerebrovascular accident, chills, cholestatis, clitorism, congestive heart failure, diplopia, edema, extrapyramidal symptoms, grand mal seizures, hallucinations, hemolytic anemia, hirsutism, hyperbilirubinemia, increased amylase, increased salivation, insomnia, leukocytosis, leukonychia, jaundice, lactation, liver enzyme alterations, methemoglobinemia, nausea/vomiting (most frequently), paresthesia, paranoid reaction, priapism (see WARNINGS and PRECAUTIONS, Information for Patients; some patients have required surgical intervention), pruritus, psoriasis, psychosis, rash, stupor, inappropriate ADH syndrome, tardive dyskinesia, unexplained death, urinary incontinence, urinary retention, urticaria, vasodilation, vertigo, and weakness. Cardiovascular system effects which have been reported include the following: conduction block orthostatic hypotension and syncope, palpitations, bradycardia, atrial fibrillation, myocardial infarction, cardiac arrest, arrhythmia, and ventricular ectopic activity, including ventricular tachycardia (see WARNINGS).
Overdosage Animal Oral LD50: The oral LD50 of the drug is 610 mg/kg in mice, 486 mg/kg in rats, and 560 mg/kg in rabbits. Signs and Symptoms: Death from overdose has occurred in patients ingesting Trazodone HCl and other drugs concurrently (namely, alcohol; alcohol + chloral hydrate + diazepam; amobarbital; chlordiazepoxide; or meprobamate).
The most severe reactions reported to have occurred with overdose of Trazodone HCl alone have been priapism, respiratory arrest, seizures, and EKG changes. The reactions reported most frequently have been drowsiness and vomiting. Overdosage may cause an increase in incidence or severity of any of the reported adverse reactions (see ADVERSE REACTIONS). Treatment There is no specific antidote for Trazodone HCl. Treatment should be symptomatic and supportive in the case of hypotension or excessive sedation. Any patient suspected of having taken an overdose should have the stomach emptied by gastric lavage. Forced diuresis may be useful in facilitating elimination of the drug.
Trazodone Dosage and Administration
The dosage should be initiated at a low level and increased gradually, noting the clinical response and any evidence of intolerance. Occurrence of drowsiness may require the administration of a major portion of the daily dose at bedtime or a reduction of dosage. Trazodone HCl should be taken shortly after a meal or light snack. Symptomatic relief may be seen during the first week with optimal antidepressant effects typically evident within two weeks. Twenty-five percent of those who respond to Trazodone HCl require more than two weeks (up to four weeks) of drug administration.
Usual Adult Dosage: An initial dose of 150 mg/day in divided doses is suggested. The dose may be increased by 50 mg/day every three to four days. The maximum dose for outpatients usually should not exceed 400 mg/day in divided doses. Inpatients (i.e., more severely depressed patients) may be given up to but not in excess of 600 mg/day in divided doses. Maintenance: Dosage during prolonged maintenance therapy should be kept at the lowest effective level. Once an adequate response has been achieved, dosage may be gradually reduced, with subsequent adjustment depending on therapeutic response. Although there has been no systematic evaluation of the efficacy of Trazodone beyond 6 weeks, it is generally recommended that a course of antidepressant drug treatment should be continued for several months.
REFERENCES (a) Williams JBW, Ed: Diagnostic and Statistical Manual of Mental Disorders lll, American Psychiatric Association, May, 1980. (b) Lue TF, Physiology of erection and pathophysiology of impotence. In: Wash PC, Retik AB, Stamey TA, Vaughan ED, eds. Campbell’s Urology. Sixth edition. Philadelphia: W.B. Saunders: 1992: 722-725. (c) Goldstein I, Krane RJ, Diagnosis and therapy of erectile dysfunction. In: Wash PC. Retik AB, Stamey TA, Vaughan ED, eds. Campbell’s Urology. Sixth edition. Philadelphia: W.B. Saunders: 1992: 3071-3072. (d) Yealy DM, Hogya PT: Priapism. Emerg Med Clin North Am, 1988: 6:509-520. (e) Banos JE, Bosch F, Farre M. Drug-induced priapism, its aetiology, incidence and treatment. Med Toxicol Adverse Drug Exp. 1989: 4:46-58. (f) O’Brien WM, O’Connor KP, Lynch JH. Priapism: current concepts. Ann Emerg Med. 1989: 980-983. (g) Bardin ED, Krieger JN. Pharmacological priapism: comparison of Trazodone- and papaverine-associated cases. Int Urol Nephrol. 1990: 22:147- 152.
Medication Guide Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions Read the Medication Guide that comes with you or your family member’s antidepressant medicine.
This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines.
Talk to your, or your family member’s, healthcare provider about: • all risks and benefits of treatment with antidepressant medicines • all treatment choices for depression or other serious mental illness What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member? • Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed. • Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings. • Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms. Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
| • thoughts about suicide or dying | • new or worse irritability |
| • attempts to commit suicide | • acting aggressive, being angry, or violent |
| • new or worse depression | • acting on dangerous impulses |
| • new or worse anxiety | • an extreme increase in activity and talking (mania) |
| • feeling very agitated or restless | • other unusual changes in behavior or mood |
| • panic attacks |
|
| • trouble sleeping (insomnia) |
|
Packaged by Bryant Ranch North Hollywood CA 91605 Trazadone 50mg Tablet Compare To Desyrel 50mg Tablet Barr Laboratories NC #30 EXP 02/09 NDC 6362915132 May cause dizziness or drowsiness Keep all drugs out of reach of children
LOT 14507
Rx Only
A convenience Packed Medical Food and Drug Trazamine PHYSICIAN THERAPEUTICS Sentra PM 60 Capsules Trazadone 50mg 30 Tablets No Refills Without Physician Authorization Rx Only NDC # 68405-8003-06 of this co-pack For the Dietary Management of Sleep Disorders. Two capsules at bedtime or as directed by physician. See product label and insert Sentra PM Medical Food As prescribed by physician. See product label and product information insert. Trazadone 50mg Rx Drug
Sentra PM™PRODUCT INFORMATION
Sentra PM (U.S. patent pending) capsules by oral administration. A specially formulated Medical Food product, consisting of a proprietary blend of amino acids and polyphenol ingredients in specific proportions, for the dietary management of the metabolic processes of sleep disorders (SD). Must be administered under physician supervision.
Medical Foods Medical Food products are often used in hospitals (e.g., for burn victims or kidney dialysis patients) and outside of a hospital setting under a physician’s care for the dietary management of diseases in patients with particular medical or metabolic needs due to their disease or condition. Congress defined "Medical Food" in the Orphan Drug Act and Amendments of 1988 as "a system which is formulated to be consumed or administered enterally [or orally] under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation." Medical Foods are complex formulated products, requiring sophisticated and exacting technology. Sentra PM has been developed, manufactured, and labeled in accordance with both the statutory and the FDA regulatory definition of a Medical Food. Sentra PM must be used while the patient is under the ongoing care of a physician.
SLEEP DISORDERS (SD)
SD as a Metabolic Deficiency Disease A critical component of the definition of a Medical Food is the requirement for a distinctive nutritional deficiency. FDA scientists have proposed a physiologic definition of a distinctive nutritional deficiency as follows: “the dietary management of patients with specific diseases requires, in some instances, the ability to meet nutritional requirements that differ substantially from the needs of healthy persons. For example, in establishing the recommended dietary allowances for general, healthy population, the Food and Nutrition Board of the Institute of Medicine National Academy of Sciences, recognized that different or distinctive physiologic requirements may exist for certain persons with "special nutritional needs arising from metabolic disorders, chronic diseases, injuries, premature birth, other medical conditions and drug therapies. Thus, the distinctive nutritional needs associated with a disease reflect the total amount needed by a healthy person to support life or maintain homeostasis, adjusted for the distinctive changes in the nutritional needs of the patient as a result of the effects of the disease process on absorption, metabolism, and excretion.” It was also proposed that in patients with certain disease states who respond to nutritional therapies, a physiologic deficiency of the nutrient is assumed to exist. For example, if a patient with sleep disorders responds to a tryptophan formulation by improving the duration and quality of sleep, a deficiency of tryptophan is assumed to exist.
Patients with sleep disorders are known to have nutritional deficiencies of tryptophan, choline, flavonoids, and certain antioxidants. Patients with sleep disorders frequently exhibit reduced plasma levels of tryptophan and have been shown to respond to oral administration of tryptophan or a 5-hydoxytryptophan formulation. Research has shown that tryptophan reduced diets result in a fall of circulating tryptophan. Patients with sleep disorders have activation of the tryptophan degradation pathway that increases the turnover of tryptophan leading to a reduced level of production of serotonin for a given tryptophan blood level. Research has also shown that a genetic predisposition can lead to increased tryptophan requirements in certain patients with sleep disorders.
Choline is required to fully potentiate acetylcholine synthesis by brain neurons. A deficiency of choline leads to reduced acetylcholine production by the neurons. Low fat diets, frequently used by patients with sleep disorders, are usually choline deficient. Flavonoids potentiate the production of acetylcholine by the neurons thereby inducing REM sleep. Low fat diets and diets deficient in flavonoid rich foods result in inadequate flavonoid concentrations, impeding acetylcholine production in certain patients with sleep disorders. Provision of tryptophan, choline, and flavonoids with antioxidants, in specific proportions can restore the production of beneficial serotonin and acetylcholine, thereby improving sleep quality.
PRODUCT DESCRIPTION
Primary Ingredients
Sentra PM consists of a proprietary blend of amino acids, cocoa, ginkgo biloba and flavonoids in specific proportions. These ingredients fall into the category of “Generally Regarded as Safe” (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the U.S. Food and Drug Administration (FDA) to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186.
Amino Acids
Amino Acids are the building blocks of protein. All amino acids are GRAS listed as they have been ingested by humans for thousands of years. The doses of the amino acids in Sentra PM are equivalent to those found in the usual human diet; however the formulation uses specific ratios of the key ingredients to elicit a therapeutic response. Patients with sleep disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to induce sleep. Patients with sleep disorders have altered serotonin metabolism. Some patients with sleep disorders have a resistance to the use of tryptophan that is similar to the mechanism found in insulin resistance that is genetically determined. Patients with sleep disorders frequently cannot acquire sufficient tryptophan from the diet without ingesting a prohibitively large amount of calories, particularly protein rich calories.
Flavonoids
Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Sentra PM cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response.
Other Ingredients
Sentra PM contains the following inactive or other ingredients, as fillers, excipients, and colorings: magnesium stearate, microcrystalline cellulose, Maltodextrin NF, gelatin (as the capsule material).
Physical Description
Sentra PM is a yellow to light brown powder. Sentra PM contains L-Glutamic Acid, 5-Hydroxytryptophan as Griffonia Seed Extract, Acetylcarnitine HCL, Choline Bitartrate, Cinnamon, Cocoa, Ginkgo Biloba, and Hawthorn Berry.
CLINICAL PHARMACOLOGY
Mechanism of Action
Sentra PM acts by restoring and maintaining the balance of the neurotransmitters, serotonin and acetylcholine, that are associated with sleep disorders.
Metabolism
The amino acids in Sentra PM are primarily absorbed by the stomach and small intestines. All cells metabolize the amino acids in Sentra PM. Circulating tryptophan and choline blood levels determine the production of serotonin and acetylcholine.
Excretion
Sentra PM is not an inhibitor of cytochrome P450 1A2, 2C9, 2C19, 2D6, or 3A4. These isoenzymes are principally responsible for 95% of all detoxification of drugs, with CYP3A4 being responsible for detoxification of roughly 50% of drugs. Amino acids do not appear to have an effect on drug metabolizing enzymes.
Uses
INDICATIONS FOR USE
Sentra PM is intended for the clinical dietary management of the metabolic processes associated with sleep disorders.
CLINICAL EXPERIENCE
The administration of Sentra PM has demonstrated significant functional improvement in the quality and quantity of sleep when used for the dietary management of the metabolic processes associated with sleep disorders. Administration of Sentra PM results in the induction and maintenance of sleep in patients with sleep disorders. Sentra PM has no effect on normal blood pressure.
PRECAUTIONS AND CONTRAINDICATIONS
Sentra PM is contraindicated in an extremely small number of patients with hypersensitivity to any of the nutritional components of Sentra PM.
ADVERSE REACTIONS
Oral supplementation with L-tryptophan or choline at high doses up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher doses — from 15 to 30 grams daily — are nausea, abdominal cramps, and diarrhea. Some patients may experience these symptoms at lower doses. The total combined amount of amino acids in each Sentra PM capsule does not exceed 400 mg.
DRUG INTERACTIONS
Sentra PM does not directly influence the pharmacokinetics of prescription drugs. Clinical experience has shown that administration of Sentra PM may allow for lowering the dose of co-administered drugs under physician supervision.
OVERDOSE
There is a negligible risk of overdose with Sentra PM as the total dosage of amino acids in a one month supply (60 capsules) is less than 24 grams. Overdose symptoms may include diarrhea, weakness, and nausea.
POST-MARKETING SURVEILLANCE
Post-marketing surveillance has shown no serious adverse reactions. Reported cases of mild rash and itching may have been associated with allergies to Sentra PM flavonoid ingredients, including cinnamon, cocoa, and chocolate. The reactions were transient in nature and subsided within 24 hours.
DOSAGE AND ADMINISTRATION
Recommended Administration For the dietary management of the metabolic processes associated with sleep disorders. Take (2) capsules daily at bedtime. An additional dose of one or two capsules may be taken after awakenings during the night. As with most amino acid formulations Sentra PM should be taken without food to increase the absorption of key ingredients.
How Supplied
Sentra PM is supplied in red and white, size 0 capsules in bottles of 60 capsules.
Physician Supervision
Sentra PM is a Medical Food product available by prescription only and must be used while the patient is under ongoing physician supervision.
Sentra PM is supplied to physicians in a recyclable plastic bottle with a child-resistant cap.
U.S. patents pending.
Manufactured by Arizona Nutritional Supplements, Inc. Chandler AZ 85225
Distributed by Physician Therapeutics LLC, Los Angeles, CA 90077. www.ptlcentral.com
© Copyright 2003-2006, Physician Therapeutics LLC, all rights reserved
NDC # 68405-1003-02
Storage
Store at room temperature, 59-86OF (15-30OC). Protect from light and moisture.
PHYSICIAN THERAPEUTICS
SENTRA PM
Medical Food
Rx only
60 Capsules
Directions for use:
Must be administered under medical supervision.
For adults only. As a Medical Food, take two (2) capsules at bedtime or as directed by your medical practitioner.
For the dietary management of sleep disorders.
Contains no added sugar, starch, wheat, yeast, preservatives, artificial color or flavor.
Storage:
Keep tightly closed in a cool dry place 8-320 C (45-900F), relative humidity, below 50%.
Warning: Keep this product out of the reach of children.
NDC# 68405-1003-02
Ingredients:
Each serving (2 capsules) contains:
Proprietary Amino Acid blend Choline Bitartrate, Glutamic Acid (L-Glutamic Acid), Cocoa Extract (fruit), Proprietary Herbal Blend Ginkgo Biloba (leaves), Griffonia Seed Extract (5-HTP), Hawthorn Berry (fruit), Acetyl L-Carnitine HCl, Dextrose
Other Ingredients: Gelatin, Cellulose, Dicalcium Phosphate, Silicon Dioxide and Vegetable Magnesium Stearate.
Distributed by:
Physician Therapeutics LLC, Los Angeles, CA 90077
www.ptlcentral.com
Patent Pending
A convenience Packed Medical Food and Drug Trazamine PHYSICIAN THERAPEUTICS Sentra PM 60 Capsules Trazadone 50mg 30 Tablets No Refills Without Physician Authorization Rx Only NDC # 68405-8003-06 of this co-pack For the Dietary Management of Sleep Disorders. Two capsules at bedtime or as directed by physician. See product label and insert Sentra PM Medical Food As prescribed by physician. See product label and product information insert. Trazadone 50mg Rx Drug


TrazamineTRAZODONE HYDROCHLORIDE, CHOLINE KIT
| ||||||||||||||||||||||||||||||||||||||||