Torsemide
Bryant Ranch Prepack
Bryant Ranch Prepack
FULL PRESCRIBING INFORMATION: CONTENTS*
- TORSEMIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TORSEMIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TORSEMIDE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
TORSEMIDE DESCRIPTION
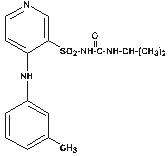
162043
CLINICAL PHARMACOLOGY
Mechanism of Action
++-
Pharmacokinetics and Metabolism
maxmaxmax
Clinical Effects
INDICATIONS & USAGE
TORSEMIDE CONTRAINDICATIONS
WARNINGS
Hepatic Disease With Cirrhosis and Ascites
Ototoxicity
Volume and Electrolyte Depletion
PRECAUTIONS
Laboratory Values
WARNINGS
Drug Interactions
Carcinogenesis & Mutagenesis & Impairment Of Fertility
in vivoin vitro
Pregnancy
22
Labor & Delivery
Nursing Mothers
Pediatric Use
Geriatric Use
TORSEMIDE ADVERSE REACTIONS
contact Hetero Labs Limited at 866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Table 1. Reactions Possibly or Probably Drug-Related United States Placebo-Controlled Studies Incidence (Percentages of Patients)
| |
Torsemide Tablets (N=564) |
Placebo (N=274) |
|
Headache |
7.3 |
9.1 |
| Excessive Urination | 6.7 |
2.2 |
| Dizziness |
3.2 |
4.0 |
| Rhinitis |
2.8 |
2.2 |
|
Asthenia |
2.0 |
1.5 |
| Diarrhea |
2.0 |
1.1 |
| ECG Abnormality |
2.0 |
0.4 |
| Cough Increase |
2.0 |
1.5 |
| Constipation |
1.8 |
0.7 |
|
Nausea |
1.8 |
0.4 |
| Arthralgia |
1.8 |
0.7 |
| Dyspespsia |
1.6 |
0.7 |
| Sore Throat |
1.6 |
0.7 |
| Myalgia |
1.6 |
1.5 |
| Chest Pain |
1.2 |
0.4 |
| Insomnia |
1.2 |
1.8 |
| Edema |
1.1 |
1.1 |
| Nervousness |
1.1 |
0.4 |
Hypokalemia: See WARNINGS
Postmarketing Experience
i.e
DOSAGE & ADMINISTRATION
General
Congestive Heart Failure
Chronic Renal Failure
Hepatic Cirrhosis
Hypertension
HOW SUPPLIED
Storage
contact Hetero Labs Limited at 866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Torsemide 10mg Tablet
TorsemideTorsemide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!