Torsemide
Torsemide Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- TORSEMIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- TORSEMIDE INDICATIONS AND USAGE
- TORSEMIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TORSEMIDE ADVERSE REACTIONS
- OVERDOSAGE
- TORSEMIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (100 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (100 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (100 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 100 mg (100 Tablet Bottle)
FULL PRESCRIBING INFORMATION
TORSEMIDE DESCRIPTION
m
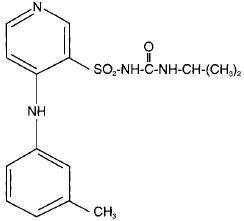
162043
CLINICAL PHARMACOLOGY
Mechanism of Action
++‾
Pharmacokinetics and Metabolism
maxmaxmax
Clinical Effects
TORSEMIDE INDICATIONS AND USAGE
TORSEMIDE CONTRAINDICATIONS
WARNINGS
Hepatic Disease With Cirrhosis and Ascites
Ototoxicity
Volume and Electrolyte Depletion
PRECAUTIONS
Laboratory Values
WARNINGS
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vivoin vitro
Pregnancy
22
Labor and Delivery
Nursing Mothers
Pediatric Use
Geriatric Use
TORSEMIDE ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
| Torsemide (N=564) |
Placebo (N=274) |
|
|---|---|---|
| Headache |
7.3 |
9.1 |
| Excessive Urination |
6.7 |
2.2 |
| Dizziness |
3.2 |
4 |
| Rhinitis |
2.8 |
2.2 |
| Asthenia |
2 |
1.5 |
| Diarrhea |
2 |
1.1 |
| ECG Abnormality |
2 |
0.4 |
| Cough Increase |
2 |
1.5 |
| Constipation |
1.8 |
0.7 |
| Nausea |
1.8 |
0.4 |
| Arthralgia |
1.8 |
0.7 |
| Dyspepsia |
1.6 |
0.7 |
| Sore Throat |
1.6 |
0.7 |
| Myalgia |
1.6 |
1.5 |
| Chest Pain |
1.2 |
0.4 |
| Insomnia |
1.2 |
1.8 |
| Edema |
1.1 |
1.1 |
| Nervousness |
1.1 |
0.4 |
Hypokalemia
WARNINGS.
Postmarketing Experience
i.e.,
OVERDOSAGE
TORSEMIDE DOSAGE AND ADMINISTRATION
General
Congestive Heart Failure
Chronic Renal Failure
Hepatic Cirrhosis
Hypertension
HOW SUPPLIED
Torsemide Tablets 5 mg
Torsemide Tablets 10 mg
Torsemide Tablets 20 mg
Torsemide Tablets 100 mg
Store at
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (100 Tablet Bottle)
NDC 65862-125-01
Torsemide Tablets
5 mg
Rx only 100 Tablets
AUROBINDO
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (100 Tablet Bottle)
NDC 65862-126-01
Torsemide Tablets
10 mg
Rx only 100 Tablets
AUROBINDO
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (100 Tablet Bottle)
NDC 65862-127-01
Torsemide Tablets
20 mg
Rx only 100 Tablets
AUROBINDO

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 100 mg (100 Tablet Bottle)
NDC 65862-128-01
Torsemide Tablets
100 mg
Rx only 100 Tablets
AUROBINDO

TorsemideTorsemide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TorsemideTorsemide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TorsemideTorsemide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TorsemideTorsemide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!