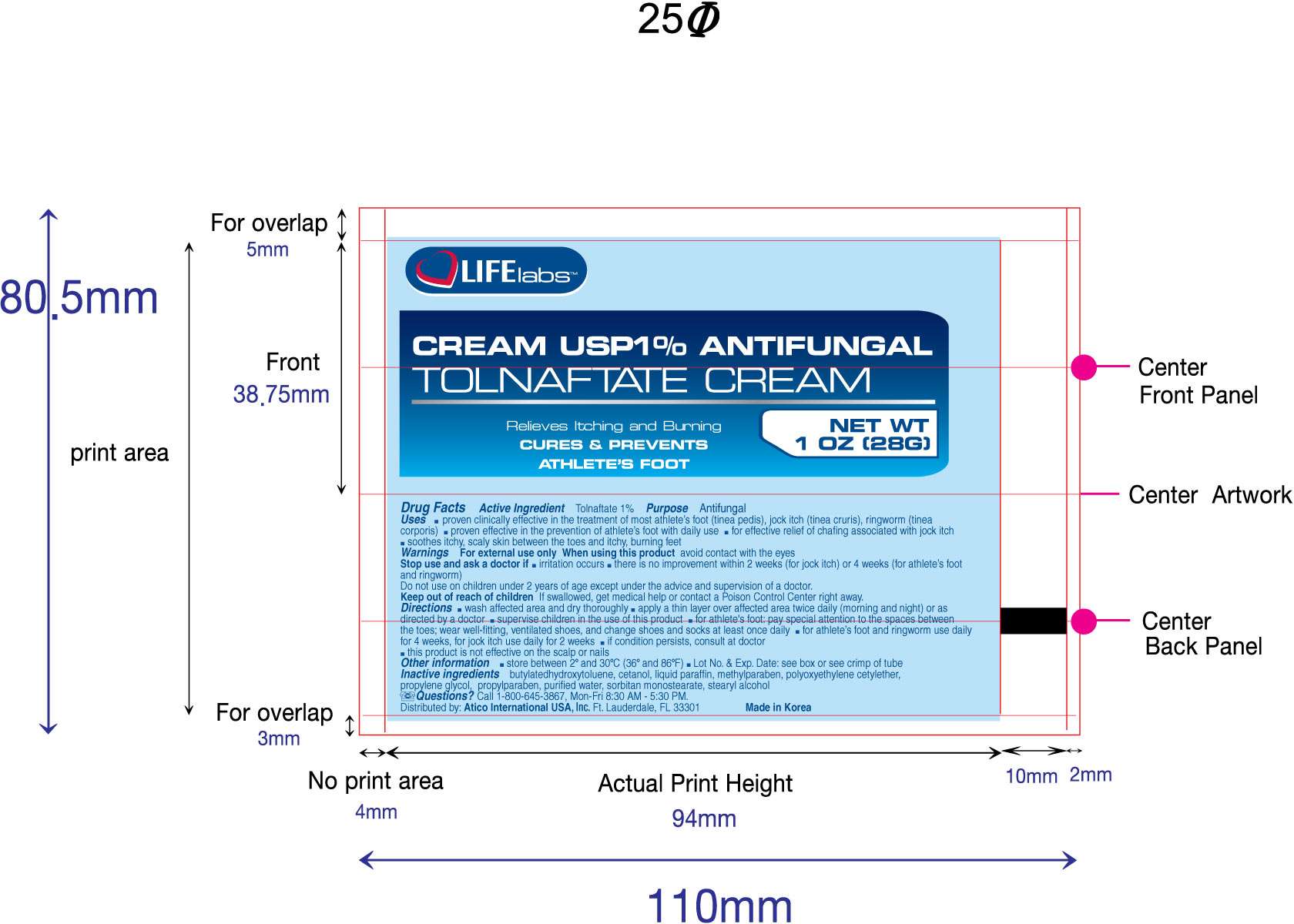
Tolnaftate
LIFElabs, a Division of Atico International, USA, INC.
LIFElabs, a Division of Atico International USA, INC.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Tolnaftate
Active ingredient
Tolnaftate 1%
Purpose
Purpose
Antifungal
Uses
- proven clinically effective in the treatment of most athlete's foot (tinea pedis), jock itch (tinea cruris), ringworm (tinea corporis)
- proven effective in the prevention of athlete's foot with daily use
- for effective relief of chafing associated with jock itch
- soothes itchy, scaly skin between the toes and itchy, burning feet
Warnings
For external use only
Uses
When using this product avoid contact with the eyes
Stop use and ask a doctor if
- irritation occurs
- there is no improvement within 2 weeks (for jock itch) or 4 weeks (for athlete's foot and ringworm)
Do not use on children under 2 years of age except under the advice and supervision of a doctor
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between the toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily
- for athlete's foot and ringworm use daily for 4 weeks, for jock itch use daily for 2 weeks
- if condition persists longer consult a doctor
- this product is not effective on the scalp or nails
Other information
store between 2o and 30oC (36o and 86oF)
Lot No. and Exp. Date: see box or see crimp of tube
Inactive ingredients
butylated hydroxytoluene, cetanol, liquid paraffin, methylparaben, polyoxyethylene cetylether, propylene glycol, propylparaben, purfied water, sorbitan monostearate, stearyl alcohol
Questions?
Call 1-800-645-3867, Mon-Fri 8:30 AM - 5:30PM.

TolnaftateTolnaftate CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||