TIAGABINE HYDROCHLORIDE
Sun Pharmaceutical Industries Limited
Tiagabine Hydrochloride Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- TIAGABINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- TIAGABINE HYDROCHLORIDE INDICATIONS AND USAGE
- TIAGABINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TIAGABINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- TIAGABINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL TOXICOLOGY
- Medication Guide
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 2MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 4MG
FULL PRESCRIBING INFORMATION
TIAGABINE HYDROCHLORIDE DESCRIPTION
202522
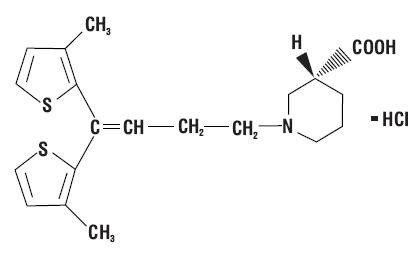
Inactive Ingredients
CLINICAL PHARMACOLOGY
Mechanism of Action
in vitro5085
in vitro1A23121A11B
Pharmacokinetics
PRECAUTIONS, General, Use in Non-Induced Patients
Absorption and Distribution:maxmax
Metabolism and Elimination:in vivoin vitro
in vitro
min
Special Populations
Renal Insufficiency:
Hepatic Insufficiency: PRECAUTIONS
Geriatric
Pediatric:
Gender, Race and Cigarette Smoking:
Interactions with other Antiepilepsy Drugs: PRECAUTIONS, Drug Interactions
Interactions with Other Drugs: See PRECAUTIONS, Drug Interactions.
CLINICAL STUDIES
| Placebo (N=91) |
Tiagabine Hydrochloride 16 mg/day (N=61) |
Tiagabine Hydrochloride 32 mg/day (N=87) |
Tiagabine Hydrochloride 56 mg/day (N=56) |
Combined 32 + 56 mg/day (N=143) | ||
|---|---|---|---|---|---|---|
| Complex Partial |
Median Reduction |
0.6 |
0.8 |
2.2 |
2.9 |
2.6 |
| Median % Reduction  |
9% |
13% |
25% |
32% |
29% |
|
| All Partial |
Median Reduction |
0.2 |
1.2 |
2.7 |
3.5 |
2.9 |
| Median % Reduction  |
3% |
12% |
24% |
36% |
27% |
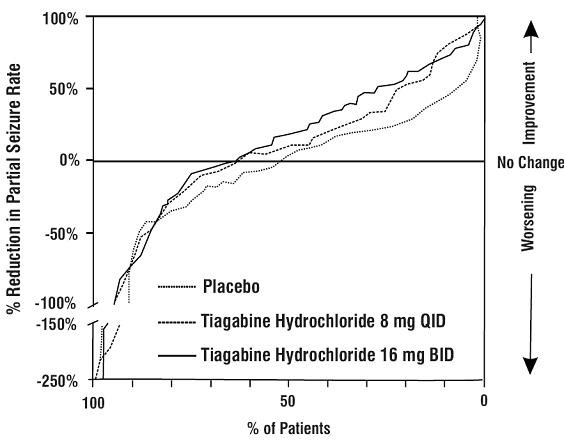
| Placebo (N=107) |
Tiagabine Hydrochloride 16 mg BID (N=106) |
Tiagabine Hydrochloride 8 mg QID (N=104) |
||
|---|---|---|---|---|
| Complex Partial |
Median Reduction |
0.3 |
1.6 |
1.3 |
Median % Reduction |
4% |
22% |
15% |
|
| All Partial |
Median Reduction |
0.5 |
1.6 |
1.3 |
Median % Reduction |
5% |
19% |
13% |
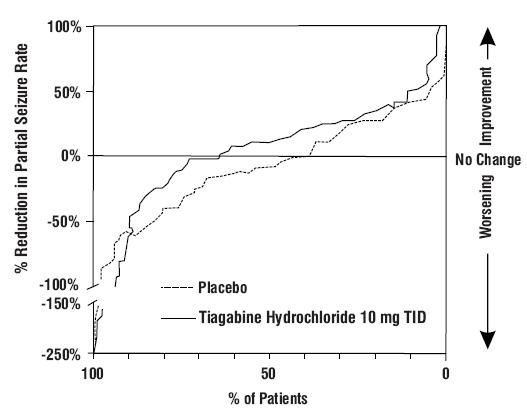
Figure 1
Study 1
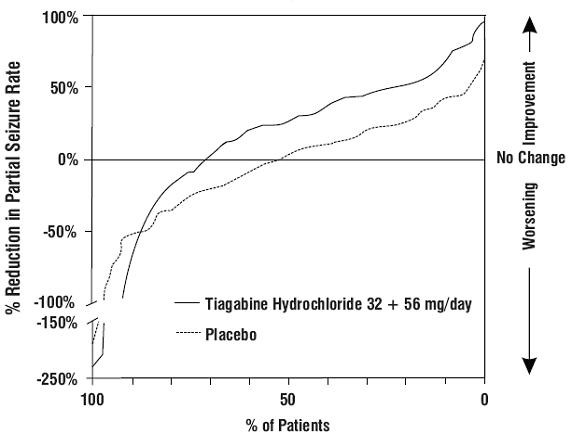
Figure 2
Study 1
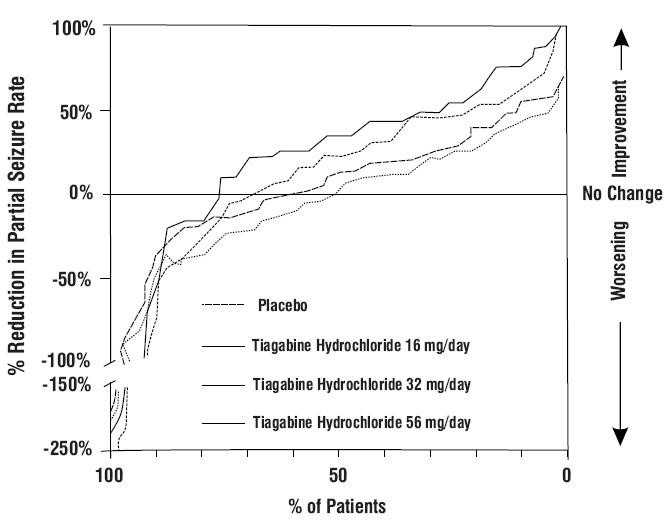
Figure 3
Study 2
| Placebo (N=77) |
Tiagabine Hydrochloride 30 mg/day (N=77) |
||
|---|---|---|---|
Complex Partial |
Median Reduction |
-0.1 |
1.3 |
Median % Reduction |
-1% |
14% |
|
| All Partial |
Median Reduction |
-0.5 |
1.1 |
Median % Reduction |
-7% |
11% |
Figure 4
Study 3
TIAGABINE HYDROCHLORIDE INDICATIONS AND USAGE
TIAGABINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Seizures in Patients Without Epilepsy: Postmarketing reports have shown that tiagabine hydrochloride use has been associated with new onset seizures and status epilepticus in patients without epilepsy. Dose may be an important predisposing factor in the development of seizures, although seizures have been reported in patients taking daily doses of tiagabine hydrochloride as low as 4 mg/day. In most cases, patients were using concomitant medications (antidepressants, antipsychotics, stimulants, narcotics) that are thought to lower the seizure threshold. Some seizures occurred near the time of a dose increase, even after periods of prior stable dosing.
The tiagabine hydrochloride dosing recommendations in current labeling for treatment of epilepsy were based on use in patients with partial seizures 12 years of age and older, most of whom were taking enzyme-inducing antiepileptic drugs (AEDs; e.g., carbamazepine, phenytoin, primidone and phenobarbital) which lower plasma levels of tiagabine hydrochloride by inducing its metabolism. Use of tiagabine hydrochloride without enzyme-inducing antiepileptic drugs results in blood levels about twice those attained in the studies on which current dosing recommendations are based (see DOSAGE AND ADMINISTRATION).
Safety and effectiveness of tiagabine hydrochloride have not been established for any indication other than as adjunctive therapy for partial seizures in adults and children 12 years and older.
In nonepileptic patients who develop seizures while on tiagabine hydrochloride treatment, tiagabine hydrochloride should be discontinued and patients should be evaluated for an underlying seizure disorder.
Seizures and status epilepticus are known to occur with tiagabine hydrochloride overdosage (see OVERDOSAGE).
Suicidal Behavior and Ideation:
|
Indication
|
Placebo Patients with Events per 1000 Patients
|
Drug Patients with Events per 1000 Patients
|
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients
|
Risk Difference: Additional Drug Patients with Events per 1000 Patients
|
| Epilepsy |
1 |
3.4 |
3.5 |
2.4 |
| Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
| Other |
1 |
1.8 |
1.9 |
0.9 |
| Total |
2.4 |
4.3 |
1.8 |
1.9 |
Withdrawal Seizures: CLINICAL STUDIES
Cognitive/Neuropsychiatric Adverse Events:
PRECAUTIONS, Laboratory Tests, EEG
Status Epilepticus:
Sudden Unexpected Death In Epilepsy (SUDEP):
PRECAUTIONS
General
Use in Non-Induced Patients: DOSAGE AND ADMINISTRATION
Generalized Weakness:
Binding in the Eye and Other Melanin-Containing Tissues:
Use in Hepatically-Impaired Patients:
Serious Rash:
Information for Patients
Suicidal Thinking and Behavior
PRECAUTIONS, Pregnancy
Laboratory Tests
Clinical Chemistry and Hematology:
WARNINGS, Cognitive/Neuropsychiatric Adverse Events
Drug Interactions
PRECAUTIONS, General, Use in Non-Induced Patients
Effects of Tiagabine Hydrochloride on other Antiepilepsy Drugs (AEDs):
Phenytoin
Carbamazepine
Valproate
Phenobarbital or Primidone:
Effects of other Antiepilepsy Drugs (AEDs) on Tiagabine Hydrochloride:
Carbamazepine
Phenytoin
Valproatein vitroin vitro
Interaction of Tiagabine Hydrochloride with Other Drugs:
Cimetidine
Theophylline
Warfarin
Digoxin
Ethanol or Triazolam:
Oral Contraceptives:
Antipyrine
Interaction of Tiagabine Hydrochloride with Highly Protein Bound Drugs:
In vitro
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:2
Mutagenesisin vitroin vitroin vitroin vivo
Impairment of Fertility:22
Pregnancy
22
22
To provide additional information regarding the effects of in utero exposure to tiagabine hydrochloride, physicians are advised to recommend that pregnant patients taking tiagabine hydrochloride enroll in the NAAED Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Use in Nursing Mothers
Pediatric Use
CLINICAL PHARMACOLOGY, Special Populations, Pediatric
Geriatric Use
TIAGABINE HYDROCHLORIDE ADVERSE REACTIONS
Adverse Event Incidence in Controlled Clinical Trials:
| Body System/ COSTART |
Tiagabine Hydrochloride N=494 % |
Placebo N=275 % |
|---|---|---|
|
Body as a Whole
|
||
| Abdominal Pain |
7 |
3 |
| Pain (unspecified) |
5 |
3 |
|
Cardiovascular
|
||
| Vasodilation |
2 |
1 |
|
Digestive
|
||
| Nausea |
11 |
9 |
| Diarrhea |
7 |
3 |
| Vomiting |
7 |
4 |
| Increased Appetite |
2 |
0 |
| Mouth Ulceration |
1 |
0 |
|
Musculoskeletal
|
||
| Myasthenia |
1 |
0 |
|
Nervous System
|
||
| Dizziness |
27 |
15 |
| Asthenia |
20 |
14 |
| Somnolence |
18 |
15 |
| Nervousness |
10 |
3 |
| Tremor |
9 |
3 |
Difficulty with Concentration/Attention |
6 |
2 |
| Insomnia |
6 |
4 |
| Ataxia |
5 |
3 |
| Confusion |
5 |
3 |
| Speech Disorder |
4 |
2 |
Difficulty with Memory |
4 |
3 |
| Paresthesia |
4 |
2 |
| Depression |
3 |
1 |
| Emotional Lability |
3 |
2 |
| Abnormal Gait |
3 |
2 |
| Hostility |
2 |
1 |
| Nystagmus |
2 |
1 |
Language Problems |
2 |
0 |
| Agitation |
1 |
0 |
|
Respiratory System
|
||
| Pharyngitis |
7 |
4 |
| Cough Increased |
4 |
3 |
|
Skin and Appendages
|
||
| Rash |
5 |
4 |
| Pruritus |
2 |
0 |
| Body System/ COSTART Term |
Tiagabine Hydrochloride 56 mg (N=57) % |
Tiagabine Hydrochloride 32 mg (N=88) % |
Placebo (N=91) % |
|---|---|---|---|
|
Body as a Whole
|
|||
| Accidental Injury |
21 |
15 |
20 |
| Infection |
19 |
10 |
12 |
| Flu Syndrome |
9 |
6 |
3 |
| Pain |
3 |
7 |
2 |
| Abdominal Pain |
5 |
7 |
4 |
|
Digestive System
|
|||
| Diarrhea |
2 |
10 |
6 |
|
Hemic and Lymphatic System
|
|||
| Ecchymosis |
0 |
6 |
1 |
|
Musculoskeletal System
|
|||
| Myalgia |
5 |
2 |
3 |
|
Nervous System
|
|||
| Dizziness |
28 |
31 |
12 |
| Asthenia |
23 |
18 |
15 |
| Tremor |
21 |
14 |
1 |
| Somnolence |
19 |
21 |
17 |
| Nervousness |
14 |
11 |
6 |
Difficulty with Concentration/Attention |
14 |
7 |
3 |
| Ataxia |
9 |
6 |
6 |
| Depression |
7 |
1 |
0 |
| Insomnia |
5 |
6 |
3 |
| Abnormal Gait |
5 |
5 |
3 |
| Hostility |
5 |
5 |
2 |
|
Respiratory System
|
|||
| Pharyngitis |
7 |
8 |
6 |
|
Special Senses
|
|||
| Amblyopia |
4 |
9 |
8 |
|
Urogenital System
|
|||
| Urinary Tract Infection |
5 |
0 |
2 |
Other Adverse Events Observed During All Clinical Trials:
Body as a Whole: FrequentInfrequent
Cardiovascular System: FrequentInfrequent
Digestive SystemFrequent Infrequent:
Endocrine SystemInfrequent
Hemic and Lymphatic System: FrequentInfrequent:
Metabolic and Nutritional: FrequentInfrequent:
Musculoskeletal System: FrequentInfrequent:
Nervous System: FrequentInfrequent
Respiratory System: FrequentInfrequent
Skin and Appendages: FrequentInfrequent
Special Senses: FrequentInfrequent
Urogenital System: FrequentInfrequent
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
Human Overdose Experience:
Management of Overdose:
TIAGABINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
General:
- Tiagabine hydrochloride tablets are given orally and should be taken with food.
- Do not use a loading dose of tiagabine hydrochloride tablets
- Dose titration: Rapid escalation and/or large dose increments of tiagabine hydrochloride tablets should not be used.
- Missed dose(s): If the patient forgets to take the prescribed dose of tiagabine hydrochloride tablets at the scheduled time, the patient should not attempt to make up for the missed dose by increasing the next dose. If a patient has missed multiple doses, patient should refer back to his or her physician for possible re-titration as clinically indicated.
- Dosage adjustment of tiagabine hydrochloride tablets should be considered whenever a change in patient's enzyme-inducing status occurs as a result of the addition, discontinuation, or dose change of the enzyme-inducing agent.
Table 7
| Initiation and Titration Schedule | Total Daily Dose | |
|---|---|---|
|
Week 1
|
Initiate at 4 mg once daily |
4 mg/day |
|
Week 2
|
Increase total daily dose by 4 mg |
8 mg/day (in two divided doses) |
|
Week 3
|
Increase total daily dose by 4 mg |
12 mg/day (in three divided doses) |
|
Week 4
|
Increase total daily dose by 4 mg |
16 mg/day (in two to four divided doses) |
|
Week 5
|
Increase total daily dose by 4 to 8 mg |
20 to 24 mg/day (in two to four divided doses) |
|
Week 6
|
Increase total daily dose by 4 to 8 mg |
24 to 32 mg/day (in two to four divided doses) |
|
Usual Adult Maintenance Dose in Induced Patients:
|
32 to 56 mg/day in two to four divided doses |
|
CLINICAL PHARMACOLOGY, Pharmacokinetics and PRECAUTIONS, General, Use in Non-Induced Patients
HOW SUPPLIED
ANIMAL TOXICOLOGY
Medication Guide
Tiagabine Hydrochloride Tablets
What is the most important information I should know about tiagabine hydrochloride tablets?
Do not stop taking tiagabine hydrochloride tablets without first talking to your healthcare provider.
Tiagabine hydrochloride tablets can cause serious side effects, including:
- Tiagabine hydrochloride tablets may cause seizures in people who do not have epilepsy. If you do not have a seizure disorder and you take tiagabine hydrochloride tablets, you may have a seizure or seizures that do not stop (status epilepticus). Call your healthcare provider right away if you have a seizure and you are not taking tiagabine hydrochloride tablets for epilepsy.
- Like other antiepileptic drugs, tiagabine hydrochloride tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop tiagabine hydrochloride tablets without first talking to a healthcare provider.
- Stopping tiagabine hydrochloride tablets suddenly can cause serious problems. If you have epilepsy and stop a seizure medicine suddenly, you may have more frequent seizures or seizures that will not stop (status epilepticus).
Who should not take tiagabine hydrochloride tablets?
What should I tell my healthcare provider before taking tiagabine hydrochloride tablets?
Before taking tiagabine hydrochloride tablets, tell your healthcare provider if you:
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have liver problems
- have a history of seizures that do not stop (status epilepticus)
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if tiagabine hydrochloride tablets can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking tiagabine hydrochloride tablets. You and your healthcare provider will decide if you should take tiagabine hydrochloride tablets while you are pregnant.
- If you become pregnant while taking tiagabine hydrochloride tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic medicines during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
- are breastfeeding or plan to breastfeed. It is not known if tiagabine passes into breast milk or if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take tiagabine hydrochloride tablets.
How should I take tiagabine hydrochloride tablets?
- Take tiagabine hydrochloride tablets exactly as your healthcare provider tells you.
- Your healthcare provider may change your dose.
- Tiagabine hydrochloride tablets should be taken with food.
- Do not stop taking tiagabine hydrochloride tablets without talking to your healthcare provider. Stopping tiagabine hydrochloride tablets suddenly can increase your chances of having a seizure or cause seizures that will not stop.
- If you miss a dose of tiagabine hydrochloride tablets, do not take 2 doses of tiagabine hydrochloride tablets at the same time. Contact your healthcare provider if you miss more than one dose.
What should I avoid while taking tiagabine hydrochloride tablets?
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking tiagabine hydrochloride tablets without first talking to your healthcare provider. Taking tiagabine hydrochloride tablets with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how tiagabine hydrochloride tablets affect you. Tiagabine hydrochloride tablets can slow your thinking and motor skills.
See “What is the most important information I should know about tiagabine hydrochloride tablets?”
Tiagabine hydrochloride tablets may cause other serious side effects including:
- seizures that can happen more often or become worse
- trouble concentrating, problems with speech and language, feeling confused, feeling sleepy and tired, and problems thinking
- weakness all over your body
- eye and vision problems
- serious rash
The most common side effects of tiagabine hydrochloride tablets include:
- dizziness
- lack of energy
- drowsiness
- nausea
- nervousness
- tremor
- stomach pain
- abnormal thinking
- difficulty with concentration or attention
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store tiagabine hydrochloride tablets?
- Store tiagabine hydrochloride tablets at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F).
- Keep tiagabine hydrochloride tablets out of light
- Keep tiagabine hydrochloride tablets dry
General Information about Tiagabine Hydrochloride Tablets
.
What are the ingredients in tiagabine hydrochloride tablets?
Active Ingredient:
Inactive Ingredients:
- 2 mg tablets: FD&C Yellow No. 6 and polysorbate 80.
- 4 mg tablets: Lactose monohydrate and D&C Yellow No. 10.
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 2MG
NDC 62756-200-83
Tiagabine Hydrochloride Tablets
2 mg
Rx only
30 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: Dispense the Medication Guide provided separately to each patient.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 4MG
NDC 62756-224-83
Tiagabine Hydrochloride Tablets
4 mg
Rx only
30 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: Dispense the Medication Guide provided separately to each patient.

TIAGABINE HYDROCHLORIDETIAGABINE HYDROCHLORIDE TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TIAGABINE HYDROCHLORIDETIAGABINE HYDROCHLORIDE TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!