Terrell
Piramal Critical Care Inc.
Piramal Critical Care Inc.
TERRELL
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERRELL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TERRELL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TERRELL ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
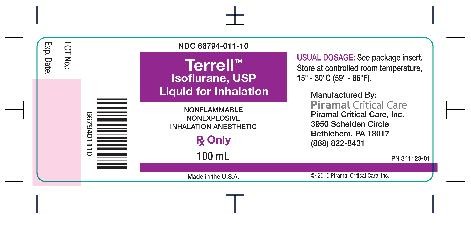
- Bottle Label - 100 ml
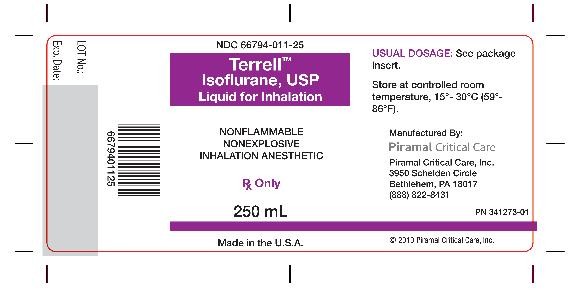
- Bottle Label - 250 ml
FULL PRESCRIBING INFORMATION
TERRELL DESCRIPTION
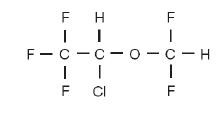
Molecular weight 184.5
Boiling point at 760 mm Hg 48.50C (uncorr.)
Refractive index n20 D 1.2990-1.3005
Specific gravity 250/250C 1.496
Vapor pressure in mm Hg** 200C 238
250C 295
300C 367
0

Partition coefficients at 37°C:
Water/gas 0.61
Blood/gas 1.43
Oil/gas 90.8
Partition coefficients at 250C - rubber and plastic
Conductive rubber/gas 62.0
Butyl rubber/gas 75.0
Polyvinyl chloride/gas 110.0
Polyurethane/gas ~1.4
Polyolefin/gas ~1.1
Butyl acetate/gas ~2.5
Purity by gas chromatography >99.9%
0
0
CLINICAL PHARMACOLOGY
Isoflurane is an inhalation anesthetic. The MAC (minimum alveolar concentration) in man is as follows:
Age 100% Oxygen 70% N2O
26 ± 4 1.28 0.56
44 ± 7 1.15 0.50
64 ± 5 1.05 0.37
Induction of and recovery from isoflurane anesthesia are rapid. Isoflurane has a mild pungency, which limits the rate of induction, although excessive salivation or tracheobronchial secretions do not appear to be stimulated. Pharyngeal and laryngeal reflexes are readily obtunded. The level of anesthesia may be changed rapidly with isoflurane. Isoflurane is a profound respiratory depressant. RESPIRATION MUST BE MONITORED CLOSELY AND SUPPORTED WHEN NECESSARY. As anesthetic dose is increased, tidal volume decreases and respiratory rate is unchanged. This depression is partially reversed by surgical stimulation, even at deeper levels of anesthesia. Isoflurane evokes a sigh response reminiscent of that seen with diethyl ether and enflurane, although the frequency is less than with enflurane.
Blood pressure decreases with induction of anesthesia but returns toward normal with surgical stimulation. Progressive increases in depth of anesthesia produce corresponding decreases in blood pressure. Nitrous oxide diminishes the inspiratory concentration of isoflurane required to reach a desired level of anesthesia and may reduce the arterial hypotension seen with isoflurane alone. Heart rhythm is remarkably stable. With controlled ventilation and normal PaC02, cardiac output is maintained despite increasing depth of anesthesia, primarily through an increase in heart rate, which compensates for a reduction in stroke volume. The hypercapnia, which attends spontaneous ventilation during isoflurane anesthesia further increases heart rate and raises cardiac output above awake levels. Isoflurane does not sensitize the myocardium to exogenously administered epinephrine in the dog. Limited data indicate that subcutaneous injection of 0.25 mg of epinephrine (50 mL of 1:200,000 solution) does not produce an increase in ventricular arrhythmias in patients anesthetized with isoflurane.
Muscle relaxation is often adequate for intra-abdominal operations at normal levels of anesthesia. Complete muscle paralysis can be attained with small doses of muscle relaxants. ALL COMMONLY USED MUSCLE RELAXANTS ARE MARKEDLY POTENTIATED WITH ISOFLURANE, THE EFFECT BEING MOST PROFOUND WITH THE NONDEPOLARIZING TYPE. Neostigmine reverses the effect of nondepolarizing muscle relaxants in the presence of isoflurane. All commonly used muscle relaxants are compatible with isoflurane.
Isoflurane can produce coronary vasodilation at the arteriolar level in selected animal models1,2; the drug is probably also a coronary dilator in humans. Isoflurane, like some other coronary arteriolar dilators, has been shown to divert blood from collateral dependent myocardium to normally perfused areas in an animal model (“coronary steal”)3. Clinical studies to date evaluating myocardial ischemia, infarction and death as outcome parameters have not established that the coronary arteriolar dilation property of isoflurane is associated with coronary steal or myocardial ischemia in patients with coronary artery disease4,5,6,7.
Pharmacokinetics
INDICATIONS & USAGE
Isoflurane may be used for induction and maintenance of general anesthesia. Adequate data have not been developed to establish its application in obstetrical anesthesia.
TERRELL CONTRAINDICATIONS
Known sensitivity to isoflurane or to other halogenated agents.
Known or suspected genetic susceptibility to malignant hyperthermia.
WARNINGS
Perioperative Hyperkalemia
Malignant Hyperthermia
22
PRECAUTIONS
General
As with any potent general anesthetic, isoflurane should only be administered in an adequately equipped anesthetizing environment by those who are familiar with the pharmacology of the drug and qualified by training and experience to manage the anesthetized patient.
Regardless of the anesthetics employed, maintenance of normal hemodynamics is important to the avoidance of myocardial ischemia in patients with coronary artery disease 4,5,6,7.
Isoflurane, like some other inhalational anesthetics, can react with desiccated carbon dioxide (CO2) absorbents to produce carbon monoxide, which may result in elevated levels of carboxyhemoglobin in some patients. Case reports suggest that barium hydroxide lime and soda lime become desiccated when fresh gases are passed through the CO2 absorber canister at high flow rates over many hours or days. When a clinician suspects that CO2 absorbent may be desiccated, it should be replaced before the administration of isoflurane .
As with other halogenated anesthetic agents, Isoflurane may cause sensitivity hepatitis in patients who have been sensitized by previous exposure to halogenated anesthetics ( See CONTRAINICATIONS ).
Information for Patients
Isoflurane, as well as other general anesthetics, may cause a slight decrease in intellectual function for 2 or 3 days following anesthesia. As with other anesthetics, small changes in moods and symptoms may persist for up to 6 days after administration.
Laboratory Tests
Transient increases in BSP retention, blood glucose and serum creatinine with decrease in BUN, serum cholesterol and alkaline phosphatase have been observed.
Drug Interactions
Isoflurane potentiates the muscle relaxant effect of all muscle relaxants, most notably nondepolarizing muscle relaxants, and MAC (minimum alveolar concentration) is reduced by concomitant administration of N2O. (See CLINICAL PHARMACOLOGY).
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Swiss ICR mice were given isoflurane to determine whether such exposure might induce neoplasia. Isoflurane was given at 1/2, 1/8 and 1/32 MAC for four in-utero exposures and for 24 exposures to the pups during the first nine weeks of life. The mice were killed at 15 months of age. The incidence of tumors in these mice was the same as in untreated control mice, which were given the same background gases, but not the anesthetic.
Pregnancy
Pregnancy Category C
Isoflurane has been shown to have a possible anesthetic-related fetotoxic effect in mice when given in doses 6 times the human dose. There are no adequate and well-controlled studies in pregnant women. Isoflurane should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when isoflurane is administered to a nursing woman.
TERRELL ADVERSE REACTIONS
WARNINGS
WARNINGS
DRUG ABUSE AND DEPENDENCE
SAFETY AND HANDLING
Occupational Caution
There is no specific work exposure limit established for Isoflurane. However, the National Institute for Occupational Safety and Health Administration (NIOSH) recommends that no worker should be exposed at ceiling concentrations greater than 2ppm of any halogenated anesthetic agent over a sampling period not to exceed one hour.
The predicted effects of acute overexposure by inhalation of Isoflurane include headache, dizziness or (in extreme cases) unconsciousness. There are no documented adverse effects of chronic exposure to halogenated anesthetic vapors (Waste Anesthetic Gases or WAGs) in the workplace. Although results of some epidemiological studies suggest a link between exposure to halogenated anesthetics and increased health problems (particularly spontaneous abortion), the relationship is not conclusive
Since exposure to WAGs is one possible factor in the findings for these studies, operating room personnel, and pregnant women in particular, should minimize exposure. Precautions include adequate general ventilation in the operating room, the use of a well-designed and well-maintained scavenging system, work practices to minimize leaks and spills while the anesthetic agent is in use, and routine equipment maintenance to minimize leaks.
STORAGE
Store at room temperature 150 to 300 C (590 to 860
OVERDOSAGE
In the event of overdosage, or what may appear to be overdosage, the following action should be taken:
Stop drug administration, establish a clear airway and initiate assisted or controlled ventilation with pure oxygen.
DOSAGE & ADMINISTRATION
Premedication
Inspired Concentration
The concentration of isoflurane being delivered from a vaporizer during anesthesia should be known. This may be accomplished by using:
a) vaporizers calibrated specifically for isoflurane;
b) vaporizers from which delivered flows can be calculated, such as vaporizers delivering a saturated vapor which is then diluted. The delivered concentration from such a vaporizer may be calculated using the formula:
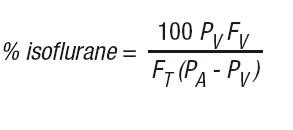
A
PV = Vapor pressure of isoflurane
FV = Flow of gas through vaporizer (mL/min)
FT = Total gas flow (mL/min)
Isoflurane contains no stabilizer. Nothing in the agent alters calibration or operation of these vaporizers.
Induction
Induction with isoflurane in oxygen or in combination with oxygen-nitrous oxide mixtures may produce coughing, breath holding, or laryngospasm. These difficulties may be avoided by the use of a hypnotic dose of an ultra-short-acting barbiturate. Inspired concentrations of 1.5 to 3.0% isoflurane usually
produce surgical anesthesia in 7 to 10 minutes.
Maintenance
Surgical levels of anesthesia may be sustained with a 1.0 to 2.5% concentration when nitrous oxide is used concomitantly. An additional 0.5 to 1.0% may be required when isoflurane is given using oxygen alone. If added relaxation is required, supplemental doses of muscle relaxants may be used.
HOW SUPPLIED
Isoflurane, USP is packaged in 100 mL and 250 mL amber-colored bottles.
100 ML - NDC 66794-011-10
250 ML - NDC 66794-011-25
REFERENCES
1. J.C. Sill, et al, Anesthesiology 66:273-279, 1987
2. R.F. Hickey, et al, Anesthesiology 68:21-30, 1988
3. C.W. Buffington, et a l, Anesthesiology 66:280-292, 1987
4. S. Reiz, et al, 59:91-97, 1983
5. S. Slogoff and A.S. Keats, Anesthesiology 70:179-188, 1989
6. K.J. Tuman, et al, Anesthesiology 70:189-198, 1989
7. D.T. Mangano, Editorial Views, Anesthesiology 70:175-178, 1989
Bottle Label - 100 ml
Bottle Label - 250 ml
TerrellIsoflurane INHALANT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||