Terbinex
Terbinex (terbinafine Hydrochloride tablets equivalent to 250mg base)
FULL PRESCRIBING INFORMATION
Terbinex™ Terbinafine Hydrochloride Tablets contain the synthetic allylamine antifungal compound terbinafine hydrochloride. Chemically, terbinafine hydrochloride is E)-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalenemethanamine hydrochloride. The empirical formula C21H26ClN with a molecular weight of 327.90, and the following structural formula:
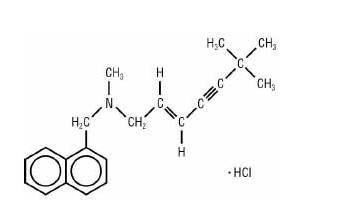
Terbinafine hydrochloride is a white to off-white fine crystalline powder. It is freely soluble in methanol and methylene chloride, soluble in ethanol, and slightly soluble in water.
Each tablet contains: Active Ingredients: terbinafine hydrochloride (equivalent to 250 mg base) Inactive Ingredients: colloidal silicon dioxide, NF; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
Following oral administration, terbinafine is well absorbed (>70%) and the bioavailability of Terbinafine Hydrochloride Tablets as a result of first-pass metabolism is approximately 40%. Peak plasma concentrations of 1 μg/mL appear within 2 h after a single 250 mg dose; the AUC (area under the curve) is approximately 4.56 μg•h/mL. An increase in the AUC of terbinafine of less than 20% is observed when terbinafine hydrochloride is administered with food. No clinically relevant age-dependent changes in steady-state plasma concentrations of terbinafine have been reported. In patients with renal impairment (creatinine clearance ≤ 50mL/min) or hepatic cirrhosis, the clearance of terbinafine is decreased by approximately 50% compared to normal volunteers. No effect of gender on the blood levels of terbinafine was detected in clinical trials. In plasma, terbinafine is >99% bound to plasma proteins and there are no specific binding sites. At steady-state, in comparison to a single dose, the peak concentration of terbinafine is 25% higher and plasma AUC increases by a factor
of 2.5; the increase in plasma AUC is consistent with an effective half-life of ~36 hours. Terbinafine is distributed to the sebum and skin. A terminal half-life of 200-400 h may represent the slow elimination of terbinafine from tissues such as skin and adipose. Prior to excretion, terbinafine is extensively metabolized. No metabolites have been identified that have antifungal activity similar to terbinafine. Approximately 70% of the administered dose is eliminated in the urine.
Terbinafine hydrochloride is a synthetic allylamine derivative. Terbinafine hydrochloride is hypothesized to act by inhibiting squalene epoxidase, thus blocking the biosynthesis of ergosterol, an essential component of fungal cell membranes. In vitro, mammalian squalene epoxidase is only inhibited at higher (4000 fold) concentrations than is needed for inhibition of the dermatophyte enzyme. Depending on the concentration of the drug and the fungal species test in vitro, terbinafine hydrochloride may be fungicidal. However, the clinical significance of in vitro data is unknown. Terbinafine has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Trichophyton mentagrophytes
Trichophyton rubrum
The following in vitro data are available, but their clinical significance is unknown. In vitro, terbinafine exhibits satisfactory MIC’s against most strains of the following microorganisms; however, the safety and efficacy of terbinafine in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials:
Candida albicans
Epidermophyton floccosum
Scopulariopsis brevicaulis
The efficacy of Terbinafine Hydrochloride Tablets in the treatment of onychomycosis is illustrated by the response of patients with toenail and/or fingernail infections who participated in three US/Canadian placebo-controlled clinical trials.
Results of the first toenail study, as assessed at week 48 (12 weeks of treatment with 36 weeks follow-up after completion of therapy), demonstrated mycological cure, defined as simultaneous occurrence of negative KOH plus negative culture, in 70% of patients. Fifty-nine percent (59%) of patients experienced effective treatment (mycological cure plus 0% nail involvement or >5mm of new unaffected nail growth); 38% of patients demonstrated mycological cure plus clinical cure (0% nail involvement).
In a second toenail study of dermatophytic onychomycosis, in which nondermatophytes were also cultured, similar efficacy against the dermatophytes was demonstrated. The pathogenic role of the non-dermatophytes cultured in the presence of dermatophytic onychomycosis has not been established. The clinical significance of this association is unknown.
Results of the fingernail study, as assessed at week 24 (6 weeks of treatment with 18 weeks follow-up after completion of therapy), demonstrated mycological cure in 79% of patients, effective treatment in 75% of the patients, and mycological cure plus clinical cure in 59% of the patients.
The mean time to overall success was approximately 10 months for the first toenail study and 4 months for the fingernail study. In the first toenail study, for patients evaluated at least six months after achieving clinical cure and at least one year after completing terbinafine hydrochloride therapy, the clinical relapse rate was approximately 15%.
Uses
Terbinafine Hydrochloride Tablets are indicated for the treatment of onychomycosis of the toenail or fingernail due to dermatophytes (tinea unguium) (see DOSAGE AND ADMINISTRATION and CLINICAL STUDIES).
Prior to initiating treatment, appropriate nail specimens for laboratory testing (KOH preparation, fungal culture, or nail biopsy) should be obtained to confirm the diagnosis of onychomycosis.
Terbinafine Hydrochloride Tablets are contraindicated in individuals with hypersensitivity to terbinafine or to any other ingredients of the formulation.
PRECAUTIONS) PRECAUTIONS
General: Terbinafine Hydrochloride Tablets are not recommended for patients with chronic or active liver disease. Before prescribing Terbinafine Hydrochloride Tablets, pre-existing liver disease should be assessed. Hepatotoxicity may occur in patients with and without pre-existing liver disease. Pretreatment serum transaminase (ALT and AST) tests are advised for all patients before taking
Terbinafine Hydrochloride Tablets. Patients prescribed Terbinafine Hydrochloride Tablets should be warned to report immediately to their physician any symptoms of persistent nausea, anorexia, fatigue, vomiting, right upper abdominal pain or jaundice, dark urine or pale stools (see WARNINGS). Patients with these symptoms should discontinue taking oral terbinafine, and the patient’s liver function should be immediately evaluated.
In patients with renal impairment (creatinine clearance ≤50 mL/ min), the use of terbinafine hydrochloride has not been adequately studied, and therefore, is not recommended (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
During postmarketing experience, precipitation and exacerbation of cutaneous and systemic lupus erythematosus have been reported infrequently in patients taking terbinafine hydrochloride. Terbinafine hydrochloride therapy should be discontinued in patients with clinical signs and symptoms suggestive of lupus erythematosus.
Changes in the ocular lens and retina have been reported following the use of Terbinafine Hydrochloride Tablets in controlled trials. The clinical significance of these changes is unknown.
Isolated cases of severe neutropenia have been reported. These were reversible upon discontinuation of terbinafine hydrochloride with or without supportive therapy. If clinical signs and symptoms suggestive of secondary infection occur, a complete blood count should be obtained. If the neutrophil count is ≤1,000 cells/mm3, terbinafine hydrochloride should be discontinued and supportive management started.
Pregnancy Category B:
The most frequently reported adverse events observed in the three US/Canadian placebo-controlled trials are listed in the table below. The adverse events reported encompass gastrointestinal symptoms (including diarrhea, dyspepsia, and abdominal pain), liver test abnormalities, rashes, urticaria, pruritus, and taste disturbances. In general, the adverse events were mild, transient, and did not lead to discontinuation from study participation.
Adverse Event DiscontinuationAbnormalities* 3.3 2.2 0.9 0.7
Total Disturbance 2.8 0.7 0.2 0.
Visual Disturbance 1.1 1.5 0.9 0.0
>
WARNINGS PRECAUTIONS
Clinical experience regarding overdose with Terbinafine Hydrochloride Tablets is limited. Doses up to 5 grams (20 times the therapeutic daily dose) have been taken without inducing serious adverse reactions. The symptoms of overdose included nausea, vomiting, abdominal pain, dizziness, rash, frequent urination,and headache.
Terbinafine Hydrochloride Tablets, one 250 mg tablet, should be taken once daily for 6 weeks by patients with fingernail onychomycosis. Terbinafine hydrochloride, one 250 mg tablet, should be taken once daily for 12 weeks by patients with toenail onychomycosis. The optimal clinical effect is seen some months after mycological cure and cessation of treatment. This is related to the period required for outgrowth of healthy nail.
Terbinex™ Terbinafine Hydrochloride Tablets
Supplied as white, round, flat faced beveled edge tablets debossed with IG on one side and 209 on the other.
Bottle of 30 tablets packaged with 12 mL bottle of Eco Formula NDC 68712-037-02
Store tablets at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]; in a tight container.
Protect from light.
Manufactured for:
Innocutis Holdings LLC
Charleston SC 29401
800-499-4468
www.jsjpharm.com
TerbinexTerbinafine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||