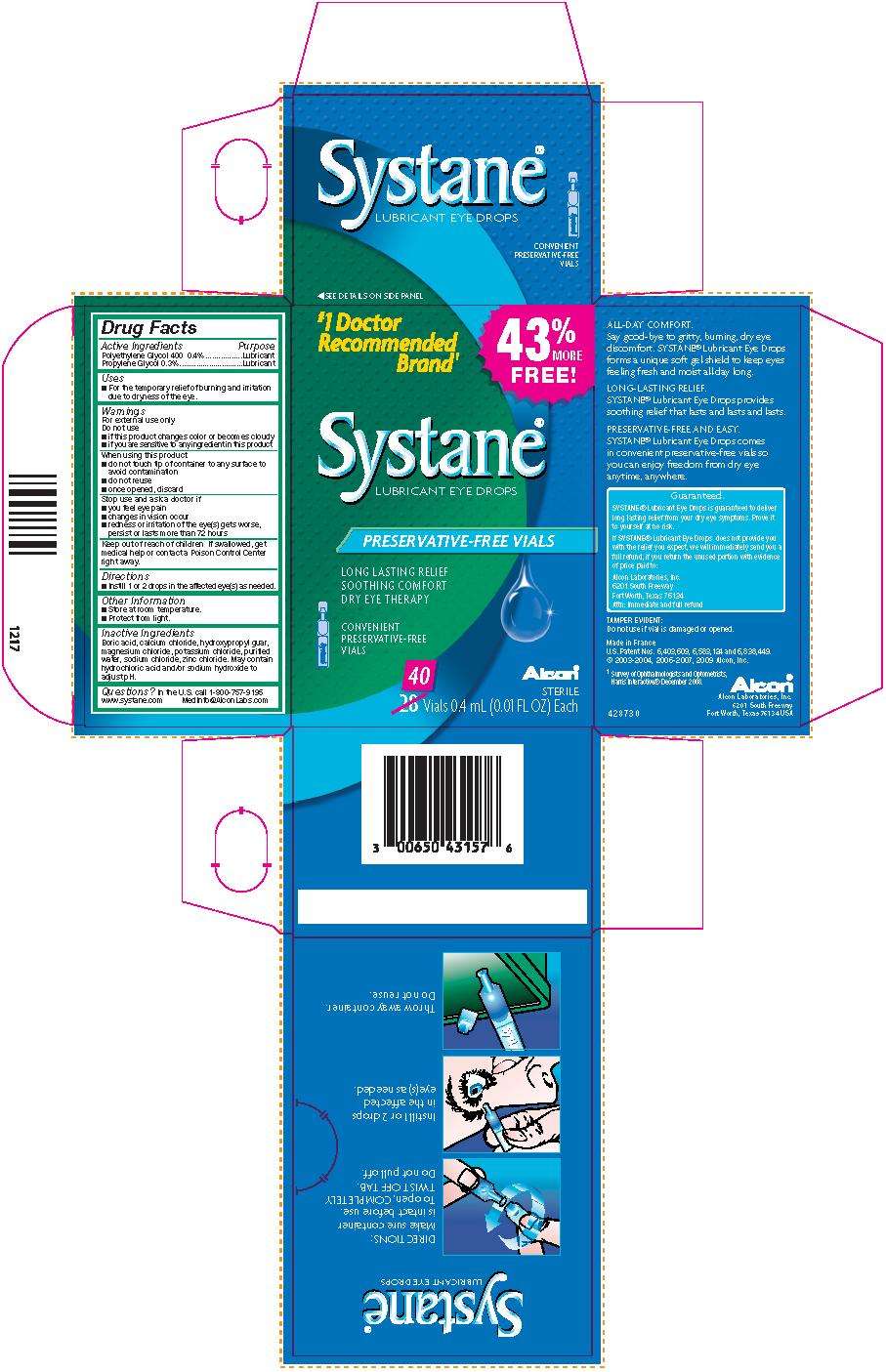
Systane
SystaneLubricant Eye Drops
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Warnings
- Systane Uses
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- LUBRICANT EYE DROPS
FULL PRESCRIBING INFORMATION
Active Ingredients
Polyethylene Glycol 400 0.4% . . . . . . . . . . . Lubricant
Propylene Glycol 0.3%. . . . . . . . . . . . . . . . . . Lubricant
Warnings
For external use only.
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- do not reuse
- once opened, discard
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse, persists or lasts more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
For the temporary relief of burning and irritation due to dryness of the eye
Directions
- Instill 1 or 2 drops in the affected eye(s) as needed.
Other Information
- Store at room temperature.
- Protect from light.
Inactive Ingredients
Boric acid, calcium chloride, hydroxypropyl guar, magnesium chloride, potassium chloride, purified water, sodium chloride, zinc chloride. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
Questions?
In the U.S. call 1-800-757-9195
www.systane.com MedInfo@AlconLabs.com
LUBRICANT EYE DROPS
43% MORE FREE!
#1 Doctor Recommended Brand1
Systane®
LUBRICANT EYE DROPS
PRESERVATIVE-FREE VIALS
LONG LASTING RELIEF
SOOTHING COMFORT
DRY EYE THERAPY
CONVENIENT PRESERVATIVE-FREE VIALS
Alcon®
STERILE
40 Vials 0.4 mL (0.01 FL OZ) Each
Systanepolyethylene glycol 0.4% and propylene glycol 0.3% SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||