SUPRANE
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Suprane (desflurane, USP) safely and effectively. See full prescribing information for Suprane (desflurane, USP). Suprane (desflurane, USP) Volatile Liquid for InhalationInitial U.S. Approval: 1992INDICATIONS AND USAGESuprane (desflurane, USP) is an inhalation agent indicated: for induction and/or maintenance of anesthesia in adults (1.1) for maintenance of anesthesia in pediatric patients following induction with agents other than Suprane (desflurane, USP) and intubation. DOSAGE AND ADMINISTRATION Suprane (desflurane, USP) should be administered only by persons trained in the administration of general anesthesia. It should only be administered using a vaporizer specifically designed and designated for use with Suprane (desflurane, USP). (2) The administration of general anesthesia must be individualized based on the patient’s response, including cardiovascular and pulmonary changes. (2) Suprane (desflurane, USP) should not be used as the sole agent for anesthetic induction in patients with coronary artery disease or where increases in heart rate or blood pressure are undesirable. (2.6) Patients with intracranial space occupying lesions (2.7) DOSAGE FORMS AND STRENGTHS Volatile liquid for inhalation, 100% Suprane (desflurane, USP) (3) CONTRAINDICATIONS Patients with known or suspected genetic susceptibility to malignant hyperthermia (4) Patients in whom general anesthesia is contraindicated (4) Induction of anesthesia in pediatric patients (4) Patients with known sensitivity to halogenated agents (4, 5.5) Patients with a history of confirmed hepatitis or with a history of unexplained moderate to severe hepatic dysfunction after anesthesia with halogenated agents (4, 5.5) WARNINGS AND PRECAUTIONS Malignant hyperthermia may occur. (5.1) Perioperative hyperkalemia may occur. Patients with latent or overt neuromuscular disease, particularly with Duchenne muscular dystrophy, appear to be most vulnerable. (5.2) Suprane (desflurane, USP) may cause sensitivity hepatitis in patients sensitized by previous exposure to halogenated anesthetics. (4, 5.5) Suprane (desflurane, USP) is not approved for maintenance of anesthesia in non-intubated children due to an increased incidence of respiratory adverse reactions. (5.3, 8.4) Suprane (desflurane, USP) may cause airway narrowing and increased airway resistance in children with Bronchial hyperactivity. (5.3) Suprane (desflurane, USP) can react with desiccated CO2 absorbents to produce carbon monoxide. (5.4) There are no adequate or well-controlled studies in pregnant or lactating women or during labor and delivery. Suprane (desflurane, USP) is a uterine relaxant. (8.1, 8.2, 8.3) Side EffectsMost common adverse reactions (incidence> 10%) are coughing, breath holding, apnea, nausea, vomiting. (6) To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare Corporation at 1-800-262-3784 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Concomitant use of N2O, benzodiazepines and/or opioids reduces the MAC of Suprane (desflurane, USP). Adjust dose accordingly. (7.1, 7.3) Suprane (desflurane, USP) decreases the doses of neuromuscular blocking agents required. Adjust dose accordingly. (7.2) Concomitant use of beta blockers may exaggerate the cardiovascular effects of inhalational anesthetics, including hypotension and negative inotropic effects. (7.4) Concomitant use of MAO inhibitors and inhalational anesthetics may increase the risk of hemodynamic instability during surgery or medical procedures. (7.5) USE IN SPECIFIC POPULATIONS Pediatric: Suprane (desflurane, USP) is contraindicated as an induction agent in children and is not indicated for maintenance of anesthesia in non-intubated children due to an increased incidence of moderate to severe respiratory adverse reactions, including coughing, laryngospasm and secretions. (5.3, 8.4) Geriatric: The minimum alveolar concentration (MAC) of Suprane (desflurane, USP) decreases with increasing patient age. (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 SUPRANE INDICATIONS AND USAGE
- 2 SUPRANE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 SUPRANE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 SUPRANE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 SUPRANE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Induction of Anesthesia
SUPRANE (desflurane, USP) is indicated as an inhalation agent for induction of anesthesia for inpatient and outpatient surgery in adults.
SUPRANE (desflurane, USP) is contraindicated as an inhalation agent for the induction of anesthesia in pediatric patients because of a high incidence of moderate to severe upper airway adverse events.
1.2 Maintenance of Anesthesia
SUPRANE (desflurane, USP) is indicated as an inhalation agent for maintenance of anesthesia for inpatient and outpatient surgery in adults and in pediatric patients.
After induction of anesthesia with agents other than SUPRANE (desflurane, USP), and tracheal intubation, SUPRANE (desflurane, USP) is indicated for maintenance of anesthesia in infants and children. SUPRANE (desflurane, USP) is not approved for maintenance of anesthesia in non-intubated children due to an increased incidence of respiratory adverse reactions, including coughing, laryngospasm, and secretions [see Warnings and Precautions (5.3) and Clinical Studies (14.5)].
2 DOSAGE AND ADMINISTRATION
Only persons trained in the administration of general anesthesia should administer SUPRANE (desflurane, USP). Only a vaporizer specifically designed and designated for use with desflurane should be utilized for its administration. Facilities for maintenance of a patent airway, artificial ventilation, oxygen enrichment, and circulatory resuscitation must be immediately available.
SUPRANE (desflurane, USP) is administered by inhalation. The administration of general anesthesia must be individualized based on the patient’s response. Hypotension and respiratory depression increase as anesthesia with SUPRANE (desflurane, USP) is deepened. The minimum alveolar concentration (MAC) of SUPRANE (desflurane, USP) decreases with increasing patient age. The MAC for SUPRANE (desflurane, USP) is also reduced by concomitant N2O administration (see Table 1). The dose should be adjusted accordingly. The following table provides mean relative potency based upon age and effect of N2O in predominately ASA physical status I or II patients.
Benzodiazepines and opioids decrease the MAC of SUPRANE (desflurane, USP) [see Drug Interactions (7.1, Table 3)]. SUPRANE (desflurane, USP) also decreases the doses of neuromuscular blocking agents required [see Drug Interactions (7.2, Table 4)]. The dose should be adjusted accordingly.
| N = number of crossover pairs (using up-and-down method of quantal response) | ||||
|
Effect of Age on Minimum Alveolar Concentration of Desflurane Mean ± SD (percent atmospheres) |
||||
| Age | N | O2 100% | N | N2O 60%/40% O2 |
| 2 weeks | 6 | 9.2 ± 0.0 | - | - |
| 10 weeks | 5 | 9.4 ± 0.4 | - | - |
| 9 months | 4 | 10.0 ± 0.7 | 5 | 7.5 ± 0.8 |
| 2 years | 3 | 9.1 ± 0.6 | - | - |
| 3 years | - | - | 5 | 6.4 ± 0.4 |
| 4 years | 4 | 8.6 ± 0.6 | - | - |
| 7 years | 5 | 8.1 ± 0.6 | - | - |
| 25 years | 4 | 7.3 ± 0.0 | 4 | 4.0 ± 0.3 |
| 45 years | 4 | 6.0 ± 0.3 | 6 | 2.8 ± 0.6 |
| 70 years | 6 | 5.2 ± 0.6 | 6 | 1.7 ± 0.4 |
2.1 Preanesthetic Medication
Issues such as whether or not to premedicate and the choice of premedication(s) must be individualized. In clinical studies, patients scheduled to be anesthetized with SUPRANE (desflurane, USP) frequently received IV preanesthetic medication, such as opioid and/or benzodiazepine.
2.2 Induction
In adults, some premedicated with opioid, a frequent starting concentration was 3% SUPRANE (desflurane, USP), increased in 0.5-1.0% increments every 2 to 3 breaths. End-tidal concentrations of 4-11%, SUPRANE (desflurane, USP) with and without N2O, produced anesthesia within 2 to 4 minutes. When SUPRANE (desflurane, USP) was tested as the primary anesthetic induction agent, the incidence of upper airway irritation (apnea, breathholding, laryngospasm, coughing and secretions) was high. During induction in adults, the overall incidence of oxyhemoglobin desaturation (SpO2 < 90%) was 6% [see Adverse Reactions (6.1)].
After induction in adults with an intravenous drug such as thiopental or propofol, SUPRANE (desflurane, USP) can be started at approximately 0.5-1 MAC, whether the carrier gas is O2 or N2O/O2.
Inspired concentrations of SUPRANE (desflurane, USP) greater than 12% have been safely administered to patients, particularly during induction of anesthesia. Such concentrations will proportionately dilute the concentration of oxygen; therefore, maintenance of an adequate concentration of oxygen may require a reduction of nitrous oxide or air if these gases are used concurrently.
2.3 Maintenance
Surgical levels of anesthesia in adults may be maintained with concentrations of 2.5-8.5% SUPRANE (desflurane, USP) with or without the concomitant use of nitrous oxide. In children, surgical levels of anesthesia may be maintained with concentrations of 5.2-10% SUPRANE (desflurane, USP) with or without the concomitant use of nitrous oxide.
During the maintenance of anesthesia with inflow rates of 2 L/min or more, the alveolar concentration of SUPRANE (desflurane, USP) will usually be within 10% of the inspired concentration [FA/FI, see Figure 2 in Clinical Pharmacology (12.3)].
During the maintenance of anesthesia, increasing concentrations of SUPRANE (desflurane, USP) produce dose-dependent decreases in blood pressure. Excessive decreases in blood pressure may be due to depth of anesthesia and in such instances may be corrected by decreasing the inspired concentration of SUPRANE (desflurane, USP).
Concentrations of SUPRANE (desflurane, USP) exceeding 1 MAC may increase heart rate. Thus with this drug, an increased heart rate may not serve reliably as a sign of inadequate anesthesia.
2.4 Maintenance of Anesthesia in Intubated Pediatric Patients
SUPRANE (desflurane, USP) is indicated for maintenance of anesthesia in infants and children after induction of anesthesia with agents other than SUPRANE (desflurane, USP), and tracheal intubation.
SUPRANE (desflurane, USP), with or without N2O, and halothane, with or without N2O were studied in three clinical trials of pediatric patients aged 2 weeks to 12 years (median 2 years) and ASA physical status I or II. The concentration of SUPRANE (desflurane, USP) required for maintenance of general anesthesia is age-dependent [see Clinical Studies (14.5)].
Changes in blood pressure during maintenance of and recovery from anesthesia with SUPRANE (desflurane, USP)/N2O/O2 are similar to those observed with halothane/N2O/O2. Heart rate during maintenance of anesthesia is approximately 10 beats per minute faster with SUPRANE (desflurane, USP) than with halothane. Patients were judged fit for discharge from post-anesthesia care units within one hour with both SUPRANE (desflurane, USP) and halothane. There were no differences in the incidence of nausea and vomiting between patients receiving SUPRANE (desflurane, USP) or halothane.
2.5 Recovery
The recovery from general anesthesia should be assessed carefully before patients are discharged from the post anesthesia care unit (PACU).
2.6 Use in Patients with Coronary Artery Disease
In patients with coronary artery disease, maintenance of normal hemodynamics is important to prevent myocardial ischemia. Marked increases in pulse rate, mean arterial pressure and levels of epinephrine and norepinephrine are associated with a rapid increase in desflurane concentrations. SUPRANE (desflurane, USP) should not be used as the sole agent for anesthetic induction in patients with coronary artery disease or patients where increases in heart rate or blood pressure are undesirable. It should be used with other medications, preferably intravenous opioids and hypnotics [see Clinical Studies (14.2)].
2.7 Neurosurgical Use
SUPRANE (desflurane, USP) may produce a dose-dependent increase in cerebrospinal fluid pressure (CSFP) when administered to patients with intracranial space occupying lesions. SUPRANE (desflurane, USP) should be administered at 0.8 MAC or less, and in conjunction with a barbiturate induction and hyperventilation (hypocapnia) until cerebral decompression in patients with known or suspected increases in CSFP. Appropriate attention must be paid to maintain cerebral perfusion pressure [see Clinical Studies (14.4)].
3 DOSAGE FORMS AND STRENGTHS
Colorless, non-flammable, volatile liquid (below 22.8°C) for inhalation, 100% desflurane.
4 CONTRAINDICATIONS
The use of SUPRANE (desflurane, USP) is contraindicated in the following conditions:
- Known or suspected genetic susceptibility to malignant hyperthermia.
- Patients in whom general anesthesia is contraindicated.
- Induction of anesthesia in pediatric patients.
- Patients with known sensitivity to SUPRANE (desflurane, USP) or to other halogenated agents [see Warnings and Precautions (5.5)].
- Patients with a history of confirmed hepatitis or with a history of unexplained moderate to severe hepatic dysfunction (e.g., jaundice associated with fever and/or eosinophilia) after anesthesia with SUPRANE (desflurane, USP) or other halogenated agents [see Warnings and Precautions (5.5)].
5 WARNINGS AND PRECAUTIONS
5.1 Malignant Hyperthermia
In susceptible individuals, potent inhalation anesthetic agents may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia. In genetically susceptible pigs, desflurane induced malignant hyperthermia. The clinical syndrome is signaled by hypercapnia, and may include muscle rigidity, tachycardia, tachypnea, cyanosis, arrhythmias, and/or unstable blood pressure. Some of these nonspecific signs may also appear during light anesthesia: acute hypoxia, hypercapnia, and hypovolemia.
Treatment of malignant hyperthermia includes discontinuation of triggering agents, administration of intravenous dantrolene sodium, and application of supportive therapy. (Consult prescribing information for dantrolene sodium intravenous for additional information on patient management.) Renal failure may appear later, and urine flow should be monitored and sustained if possible.
Fatal outcome of malignant hyperthermia has been reported with desflurane.
5.2 Perioperative Hyperkalemia
Use of inhaled anesthetic agents has been associated with rare increases in serum potassium levels that have resulted in cardiac arrhythmias and death in pediatric patients during the postoperative period. Patients with latent as well as overt neuromuscular disease, particularly Duchenne muscular dystrophy, appear to be most vulnerable. Concomitant use of succinylcholine has been associated with most, but not all, of these cases. These patients also experienced significant elevations in serum creatinine kinase levels and, in some cases, changes in urine consistent with myoglobinuria. Despite the similarity in presentation to malignant hyperthermia, none of these patients exhibited signs or symptoms of muscle rigidity or hypermetabolic state. Early and aggressive intervention to treat the hyperkalemia and resistant arrhythmias is recommended, as is subsequent evaluation for latent neuromuscular disease.
5.3 Respiratory Side Effects in Pediatric Patients
Due to the limited data available in non-intubated pediatric patients, SUPRANE (desflurane, USP) is not approved for maintenance of anesthesia in non-intubated children due to an increased incidence of respiratory adverse reactions, including coughing, laryngospasm and secretions [see Clinical Studies (14.5)].
Caution should be exercised should SUPRANE (desflurane, USP) be used for maintenance of anesthesia with laryngeal mask airway (LMA™ mask) in children, in particular for children 6 years old or younger because of the increased potential for adverse respiratory reactions, e.g. coughing and laryngospasm, especially with removal of the LMA™ mask under deep anesthesia [see Clinical Studies (14.5)].
Caution should be exercised when SUPRANE (desflurane, USP) is used for maintenance of anesthesia in children with asthma or a history of recent upper airway infection due to the potential for airway narrowing and increases in airway resistance.
5.4 Interactions with Desiccated Carbon Dioxide Absorbents
Desflurane like some other inhalation anesthetics, can react with desiccated carbon dioxide (CO2) absorbents to produce carbon monoxide that may result in elevated levels of carboxyhemoglobin in some patients. Case reports suggest that barium hydroxide lime and soda lime become desiccated when fresh gases are passed through the CO2 canister at high flow rates over many hours or days. When a clinician suspects that CO2 absorbent may be desiccated, it should be replaced before the administration of SUPRANE (desflurane, USP).
5.5 Hepatobiliary Disorders
With the use of halogenated anesthetics, disruption of hepatic function, icterus and fatal liver necrosis have been reported; such reactions appear to indicate hypersensitivity. As with other halogenated anesthetic agents, SUPRANE (desflurane, USP) may cause sensitivity hepatitis in patients who have been sensitized by previous exposure to halogenated anesthetics [see Contraindications (4)]. Cirrhosis, viral hepatitis or other pre-existing hepatic disease may be a reason to select an anesthetic other than a halogenated anesthetic. As with all halogenated anesthetics, repeated anesthesia within a short period of time should be approached with caution.
5.6 Laboratory Findings
Transient elevations in glucose and white blood cell count may occur as with use of other anesthetic agents.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Adverse event information is derived from controlled clinical trials, the majority of which were conducted in the United States. The studies were conducted using a variety of premedications, other anesthetics, and surgical procedures of varying length. Most adverse events reported were mild and transient, and may reflect the surgical procedures, patient characteristics (including disease) and/or medications administered.
Of the 2,143 patients exposed to SUPRANE (desflurane, USP) in clinical trials, 370 adults and 152 children were induced with desflurane alone and 987 patients were maintained principally with desflurane. The frequencies given reflect the percent of patients with the event. Each patient was counted once for each type of adverse event. They are presented in alphabetical order according to body system.
|
Frequency of Events Occurring in Greater Than 1% of Clinical Trial Patients (in Reports Deemed “Probably Causally Related”) |
|
|
Induction (use as a mask inhalation agent) |
|
|
Adult Patients (N=370): |
Coughing 34%, breathholding 30%, apnea 15%, increased secretions    |
|
Maintenance or Recovery Adult and Intubated Pediatric Patients (N=687): |
|
| Body as a Whole | Headache |
| Cardiovascular | Bradycardia, hypertension, nodal arrhythmia, tachycardia |
| Digestive | Nausea 27%, vomiting 16% |
| Nervous system | Increased salivation |
| Respiratory | Apnea   |
| Special Senses | Conjunctivitis (conjunctival hyperemia) |
Frequency of Events Occurring in Less Than 1% of Patients
(in Reports Deemed “Probably Causally Related”)
Reported in 3 or more patients, regardless of severity
Adverse reactions reported only from postmarketing experience or in the literature, not seen in clinical trials, are considered rare and are italicized.
| Cardiovascular | Arrhythmia, bigeminy, abnormal electrocardiogram, myocardial ischemia, vasodilation |
| Digestive | Hepatitis |
| Nervous System | Agitation, dizziness |
| Respiratory | Asthma, dyspnea, hypoxia |
|
Frequency of Events Occurring in Less Than 1% of Clinical Trial Patients
Reported in 3 or more patients, regardless of severity
|
|
| Body as a Whole | Fever |
| Cardiovascular | Hemorrhage, myocardial infarction |
| Metabolic and Nutrition | Increased creatinine phosphokinase |
| Musculoskeletal System | Myalgia |
| Skin and Appendages | Pruritus |
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of SUPRANE (desflurane, USP). Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Coagulopathy
Metabolism and Nutrition Disorders: Hyperkalemia, Hypokalemia, metabolic acidosis
Nervous System Disorders: Convulsion
Eye Disorders: Ocular icterus
Cardiac Disorders: Cardiac arrest, Torsade de pointes, ventricular failure, ventricular hypokinesia, Atrial fibrillation
Vascular Disorders: Malignant hypertension, hemorrhage, hypotension, shock
Respiratory, Thoracic and Mediastinal Disorders: Respiratory arrest, respiratory failure, respiratory distress, bronchospasm, hemoptysis
Gastrointestinal Disorders: Pancreatitis acute, abdominal pain
Hepatobiliary Disorders: Hepatic failure, hepatic necrosis, Hepatitis, cytolytic hepatitis, cholestasis, jaundice, hepatic function abnormal, liver disorder
Skin and Subcutaneous Tissue Disorder: Urticaria, erythema
Musculoskeletal, Connective Tissue and Bone Disorders: Rhabdomyolysis
General Disorders and Administration Site Conditions: Hyperthermia malignant, asthenia, malaise
Investigations: Electrocardiogram ST-T change, electrocardiogram T-wave inversion, tranaminases increased, alanine aminotransferase increased, aspartate aminotransferase increased, Blood bilirubin increased, coagulation test abnormal, ammonia increased
Injury, Poisoning, and Procedural Complications *: Tachyarrhythmia, palpitations, eye burns, blindness transient, encephalopathy, ulcerative keratitis, ocular hyperemia, visual acuity reduced, eye irritation, eye pain, dizziness, migraine, fatigue, accidental exposure, skin burning sensation, drug administration error
*All of reactions categorized within this SOC were accidental exposures to non-patients.
7 DRUG INTERACTIONS
No clinically significant adverse interactions with commonly used preanesthetic drugs, or drugs used during anesthesia (muscle relaxants, intravenous agents, and local anesthetic agents) were reported in clinical trials. The effect of SUPRANE (desflurane, USP) on the disposition of other drugs has not been determined. Similar to isoflurane, SUPRANE (desflurane, USP) does not predispose to premature ventricular arrhythmias in the presence of exogenously infused epinephrine in swine.
7.1 Benzodiazepines and Opioids (MAC Reduction)
Benzodiazepines and opioids decrease the amount of desflurane (MAC) needed to produce anesthesia. This effect is shown in Table 3 for intravenous midazolam (25-50 µg/kg) and intravenous fentanyl (3-6 µg/kg) in patients of two different age groups.
|
SUPRANE (desflurane, USP) MAC with Fentanyl or Midazolam Mean ± SD (percent reduction) |
||
| Dose | 18-30 years | 31-65 years |
| No fentanyl | 6.4 ± 0.0 | 6.3 ± 0.4 |
| 3 µg/kg fentanyl | 3.5 ± 1.9 (46%) | 3.1 ± 0.6 (51%) |
| 6 µg/kg fentanyl | 3.0 ± 1.2 (53%) | 2.3 ± 1.0 (64%) |
| No midazolam | 6.9 ± 0.1 | 5.9 ± 0.6 |
| 25 µg/kg midazolam | - | 4.9 ± 0.9 (16%) |
| 50 µg/kg midazolam | - | 4.9 ± 0.5 (17%) |
7.2 Neuromuscular Blocking Agents
Anesthetic concentrations of desflurane at equilibrium (administered for 15 or more minutes before testing) reduced the ED95 of succinylcholine by approximately 30% and that of atracurium and pancuronium by approximately 50% compared to N2O/opioid anesthesia (see Table 4). The effect of desflurane on duration of nondepolarizing neuromuscular blockade has not been studied.
|
Dosage of Muscle Relaxant Causing 95% Depression in Neuromuscular Blockade |
||||
| Desflurane Concentration | Mean ED95 (µg/kg) | |||
| Pancuronium | Atracurium | Succinylcholine | Vecuronium | |
| 0.65 MAC 60% N2O/O2 | 26 | 133 | - | - |
| 1.25 MAC 60% N2O/O2 | 18 | 119 | - | - |
| 1.25 MAC O2 | 22 | 120 | 360 | 19 |
Dosage reduction of neuromuscular blocking agents during induction of anesthesia may result in delayed onset of conditions suitable for endotracheal intubation or inadequate muscle relaxation, because potentiation of neuromuscular blocking agents requires equilibration of muscle with the delivered partial pressure of SUPRANE (desflurane, USP).
Among nondepolarizing drugs, pancuronium, atracurium, and vecuronium interactions have been studied. In the absence of specific guidelines:
- For endotracheal intubation, do not reduce the dose of nondepolarizing muscle relaxants or succinylcholine.
- During maintenance of anesthesia, the dose of nondepolarizing muscle relaxants is likely to be reduced compared to that during N2O/opioid anesthesia. Administration of supplemental doses of muscle relaxants should be guided by the response to nerve stimulation.
7.3 Concomitant use with NO
Concomitant administration of N2O reduces the MAC of SUPRANE (desflurane, USP) [see Dosage and Administration (2), Table 1].
7.4 Beta Blockers
Concomitant use of beta blockers may exaggerate the cardiovascular effects of inhalational anesthetics, including hypotension and negative inotropic effects.
7.5 Monoamine Oxidase Inhibitors (MAO)
Concomitant use of MAO inhibitors and inhalational anesthetics may increase the risk of hemodynamic instability during surgery or medical procedures.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B.
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Reproduction studies have been performed in rats at doses up to at 1 MAC hour for a minimum of 21 days and have revealed no evidence of impaired fertility or harm to the fetus due to SUPRANE (desflurane, USP).
No teratogenic effect was observed at approximately 10 and 13 cumulative MAC-Hour exposures at 1 MAC-Hour per day during organogenesis in rats or rabbits. At higher doses increased incidences of post-implantation loss and maternal toxicity were observed. However, at 10 MAC-Hours cumulative exposure in rats, about 6% decrease in the weight of male pups was observed at preterm caesarean delivery.
Rats exposed to SUPRANE (desflurane, USP) at 1 MAC-Hour per day from gestation day 15 to lactation day 21, did not show signs of dystocia. Body weights of pups delivered by these dams at birth and during lactation were comparable to that of control pups. No treatment related behavioral changes were reported in these pups during lactation.
8.2 Labor and Delivery
The safety of SUPRANE (desflurane, USP) during labor or delivery has not been demonstrated. SUPRANE (desflurane, USP) is a uterine-relaxant.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SUPRANE (desflurane, USP) is administered to a nursing woman.
8.4 Pediatric Use
Respiratory Adverse Reactions in Pediatric Patients
SUPRANE (desflurane, USP) is indicated for maintenance of anesthesia in infants and children after induction of anesthesia with agents other than SUPRANE (desflurane, USP), and tracheal intubation.
Due to limited data available in non-intubated pediatric patients, SUPRANE (desflurane, USP) is not approved for maintenance of anesthesia in non-intubated children due to an increased incidence of respiratory adverse reactions, including coughing (26%), laryngospasm (13%) and secretions (12%) [see Clinical Studies (14.5)].
Caution should be exercised should SUPRANE (desflurane, USP) be used for maintenance anesthesia with laryngeal mask airway (LMA™ mask) in children, in particular for children 6 years old or younger because of the increased potential for adverse respiratory reactions, e.g. coughing and laryngospasm, especially with removal of the LMA™ mask under deep anesthesia [see Clinical Studies (14.5)].
Caution should be exercised when SUPRANE (desflurane, USP) is used for maintenance of anesthesia in children with asthma or a history of recent upper airway infection due to the potential for airway narrowing and increases in airway resistance.
8.5 Geriatric Use
The minimum alveolar concentration (MAC) of SUPRANE (desflurane, USP) decreases with increasing patient age. The dose should be adjusted accordingly. The average MAC for SUPRANE (desflurane, USP) in a 70 year old patient is two-thirds the MAC for a 20 year old patient [see Dosage and Administration (2) Table 1 and Clinical Studies (14.3)].
8.6 Renal Impairment
Concentrations of 1-4% SUPRANE (desflurane, USP) in nitrous oxide/oxygen have been used in patients with chronic renal or hepatic impairment and during renal transplantation surgery.
Because of minimal metabolism, a need for dose adjustment in patients with renal and hepatic impairment is not to be expected.
Nine patients receiving desflurane (N=9) were compared to 9 patients receiving isoflurane, all with chronic renal insufficiency (serum creatinine 1.5-6.9 mg/dL). No differences in hematological or biochemical tests, including renal function evaluation, were seen between the two groups. Similarly, no differences were found in a comparison of patients receiving either desflurane (N=28) or isoflurane (N=30) undergoing renal transplant.
8.7 Hepatic Impairment
Eight patients receiving SUPRANE (desflurane, USP) were compared to six patients receiving isoflurane, all with chronic hepatic disease (viral hepatitis, alcoholic hepatitis, or cirrhosis). No differences in hematological or biochemical tests, including hepatic enzymes and hepatic function evaluation, were seen.
10 OVERDOSAGE
The symptoms of overdosage of SUPRANE (desflurane, USP) can present as a deepening of anesthesia, cardiac and/or respiratory depression in spontaneously breathing patients, and cardiac depression in ventilated patients in whom hypercapnia and hypoxia may occur only at a late stage. In the event of overdosage, or suspected overdosage, take the following actions: discontinue administration of SUPRANE (desflurane, USP), maintain a patent airway, initiate assisted or controlled ventilation with oxygen, and maintain adequate cardiovascular function.
11 DESCRIPTION
SUPRANE (desflurane, USP), a nonflammable liquid administered via vaporizer, is a general inhalation anesthetic. It is (±)1,2,2,2-tetrafluoroethyl difluoromethyl ether:
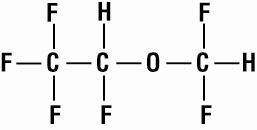
Some physical constants are:
| Molecular weight | 168.04 |
| Specific gravity (at 20°C/4°C) | 1.465 |
| Vapor pressure in mm Hg | 669 mm Hg @ 20°C |
| 731 mm Hg @ 22°C | |
|
757 mm Hg @ 22.8°C (boiling point;1atm) |
|
| 764 mm Hg @ 23°C | |
| 798 mm Hg @ 24°C | |
| 869 mm Hg @ 26°C |
Partition coefficients at 37°C:
| Blood/Gas | 0.424 |
| Olive Oil/Gas | 18.7 |
| Brain/Gas | 0.54 |
Mean Component/Gas Partition Coefficients:
| Polypropylene (Y piece) | 6.7 |
| Polyethylene (circuit tube) | 16.2 |
| Latex rubber (bag) | 19.3 |
| Latex rubber (bellows) | 10.4 |
| Polyvinylchloride (endotracheal tube) | 34.7 |
SUPRANE (desflurane, USP) is nonflammable as defined by the requirements of International Electrotechnical Commission 601-2-13.
SUPRANE (desflurane, USP) is a colorless, volatile liquid below 22.8°C. Data indicate that SUPRANE (desflurane, USP) is stable when stored under normal room lighting conditions according to instructions.
SUPRANE (desflurane, USP) is chemically stable. The only known degradation reaction is through prolonged direct contact with soda lime producing low levels of fluoroform (CHF3). The amount of CHF3 obtained is similar to that produced with MAC-equivalent doses of isoflurane. No discernible degradation occurs in the presence of strong acids.
SUPRANE (desflurane, USP) does not corrode stainless steel, brass, aluminum, anodized aluminum, nickel plated brass, copper, or beryllium.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Changes in the clinical effects of SUPRANE (desflurane, USP) rapidly follow changes in the inspired concentration. The duration of anesthesia and selected recovery measures for SUPRANE (desflurane, USP) are given in the following tables:
In 178 female outpatients undergoing laparoscopy, premedicated with fentanyl (1.5-2.0 µg/kg), anesthesia was initiated with propofol 2.5 mg/kg, desflurane/N2O 60% in O2 or desflurane/O2 alone. Anesthesia was maintained with either propofol 1.5-9.0 mg/kg/hr, desflurane 2.6-8.4% in N2O 60% in O2, or desflurane 3.1-8.9% in O2.
|
Emergence and Recovery After Outpatient Laparoscopy 178 Females, Ages 20-47 Times in Minutes: Mean ± SD (Range) |
||||
| Induction: | Propofol | Propofol | Desflurane /N2O | Desflurane /O2 |
| Maintenance: | Propofol/N2O | Desflurane /N2O | Desflurane /N2O | Desflurane /O2 |
| Number of Pts: | N = 48 | N = 44 | N = 43 | N = 43 |
| Median age | 30 (20 - 43) |
26 (21 - 47) |
29 (21 - 42) |
30 (20 - 40) |
| Anesthetic time | 49 ± 53 (8 - 336) |
45 ± 35 (11 - 178) |
44 ± 29 (14 - 149) |
41 ± 26 (19 - 126) |
| Time to open eyes | 7 ± 3 (2 - 19) |
5 ± 2 (2 - 10) |
5 ± 2 (2 - 12) |
4 ± 2 (1 - 11) |
| Time to state name | 9 ± 4 (4 - 22) |
8 ± 3 (3 - 18) |
7 ± 3 (3 - 16) |
7 ± 3 (2 - 15) |
| Time to stand | 80 ± 34 (40 - 200) |
86 ± 55 (30 - 320) |
81 ± 38 (35 - 190) |
77 ± 38 (35 - 200) |
| Time to walk | 110 ± 6 (47 - 285) |
122 ± 85 (37 – 375) |
108 ± 59 (48 - 220) |
108 ± 66 (49 - 250) |
| Time to fit for discharge | 152 ± 75 (66 - 375) |
157 ± 80 (73 - 385) |
150 ± 66 (68 - 310) |
155 ± 73 (69 - 325) |
In 88 unpremedicated outpatients, anesthesia was initiated with thiopental 3-9 mg/kg or desflurane in O2. Anesthesia was maintained with isoflurane 0.7-1.4% in N2O 60%, desflurane 1.8-7.7% in N2O 60%, or desflurane 4.4-11.9% in O2.
|
Emergence and Recovery Times in Outpatient Surgery 46 Males, 42 Females, Ages 19-70 Times in Minutes: Mean ± SD (Range) |
||||
| Induction: | Thiopental | Thiopental | Thiopental | Desflurane /O2 |
| Maintenance: | Isoflurane/N2O | Desflurane /N2O | Desflurane /O2 | Desflurane /O2 |
| Number of Pts: | N = 23 | N = 21 | N = 23 | N = 21 |
| Median age | 43 (20 - 70) |
40 (22 - 67) |
43 (19 - 70) |
41 (21-64) |
| Anesthetic time | 49 ± 23 (11 - 94) |
50 ± 19 (16 - 80) |
50 ± 27 (16 - 113) |
51 ± 23 (19 - 117) |
| Time to open eyes | 13 ± 7 (5 - 33) |
9 ± 3 (4 - 16) |
12 ± 8 (4 - 39) |
8 ± 2 (4 - 13) |
| Time to state name | 17 ± 10 (6 - 44) |
11 ± 4 (6 - 19) |
15 ± 10 (6 - 46) |
9 ± 3 (5 - 14) |
| Time to walk | 195 ± 67 (124 - 365) |
176 ± 60 (101 - 315) |
168 ± 34 (119 - 258) |
181 ± 42 (92 - 252) |
| Time to fit for discharge | 205 ± 53 (153 - 365) |
202 ± 41 (144 - 315) |
197 ± 35 (155 - 280) |
194 ± 37 (134 - 288) |
Recovery from anesthesia was assessed at 30, 60, and 90 minutes following 0.5 MAC desflurane (3%) or isoflurane (0.6%) in N2O 60% using subjective and objective tests. At 30 minutes after anesthesia, only 43% of patients in the isoflurane group were able to perform the psychometric tests compared to 76% in the SUPRANE (desflurane, USP) group (p < 0.05).
|
Recovery Tests: Percent of Preoperative Baseline Values |
||||
|
16 Males, 22 Females, Ages 20-65 |
||||
| 60 minutes After Anesthesia | 90 minutes After Anesthesia | |||
| Maintenance: | Desflurane/N20 | Isoflurane/N2O | Desflurane/N2O | Isoflurane/N2O |
Confusion  |
66 ± 6 | 47 ± 8 | 75 ± 7 |
56 ± 8 |
Fatigue  |
70 ± 9 |
33 ± 6 | 89 ± 12 |
47 ± 8 |
Drowsiness  |
66 ± 5 |
36 ± 8 | 76 ± 7 |
49 ± 9 |
Clumsiness  |
65 ± 5 | 49 ± 8 | 80 ± 7 |
57 ± 9 |
Comfort  |
59 ± 7 |
30 ± 6 | 60 ± 8 |
31 ± 7 |
DSST |
74 ± 4 |
50 ± 9 | 75 ± 4 |
55 ± 7 |
Trieger Tests |
67 ± 5 | 74 ± 6 | 90 ± 6 | 83 ± 7 |
SUPRANE (desflurane, USP) was studied in twelve volunteers receiving no other drugs. Hemodynamic effects during controlled ventilation (PaCO2 38 mm Hg) were:
|
Hemodynamic Effects of Desflurane During Controlled Ventilation |
||||||||
|
12 Male Volunteers, Ages 16-26 |
||||||||
|
Heart Rate (beats/min) |
Mean Arterial |
Cardiac Index (L/min/m2) |
||||||
|
Total MAC Equivalent |
End-Tidal % Des/O2 | End-Tidal % Des/N2O | O2 | N2O | O2 | N2O | O2 | N2O |
| 0 | 0% / 21% | 0% / 0% | 69 ± 4 (63 - 76) |
70 ± 6 (62 - 85) |
85 ± 9 (74 - 102) |
85 ± 9 (74 - 102) |
3.7 ± 0.4 (3.0 - 4.2) |
3.7 ± 0.4 (3.0 - 4.2) |
| 0.8 | 6% / 94% | 3% / 60% | 73 ± 5 (67 - 80) |
77 ± 8 (67 - 97) |
61 ± 5 (55 - 70) |
69 ± 5 (62 - 80) |
3.2 ± 0.5 (2.6 - 4.0) |
3.3 ± 0.5 (2.6 - 4.1) |
| 1.2 | 9% / 91% | 6% / 60% | 80 ± 5 (72 - 84) |
77 ± 7 (67 - 90) |
59 ± 8 (44 - 71) |
63 ± 8 (47 - 74) |
3.4 ± 0.5 (2.6 - 4.1) |
3.1 ± 0.4 (2.6 - 3.8) |
| 1.7 | 12% / 88% | 9% / 60% | 94 ± 14 (78 - 109) |
79 ± 9 (61 - 91) |
51 ± 12 (31 - 66) |
59 ± 6 (46 - 68) |
3.5 ± 0.9 (1.7 - 4.7) |
3.0 ± 0.4 (2.4 - 3.6) |
When the same volunteers breathed spontaneously during desflurane anesthesia, systemic vascular resistance and mean arterial blood pressure decreased; cardiac index, heart rate, stroke volume, and central venous pressure (CVP) increased compared to values when the volunteers were conscious. Cardiac index, stroke volume, and CVP were greater during spontaneous ventilation than during controlled ventilation.
During spontaneous ventilation in the same volunteers, increasing the concentration of SUPRANE (desflurane, USP) from 3% to 12% decreased tidal volume and increased arterial carbon dioxide tension and respiratory rate. The combination of N2O 60% with a given concentration of desflurane gave results similar to those with desflurane alone. Respiratory depression produced by desflurane is similar to that produced by other potent inhalation agents.
The use of desflurane concentrations higher than 1.5 MAC may produce apnea.
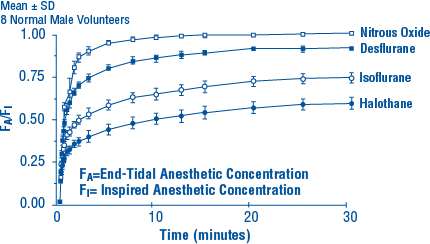
12.3 Pharmacokinetics
Due to the volatile nature of desflurane in plasma samples, the washin-washout profile of desflurane was used as a surrogate of plasma pharmacokinetics. SUPRANE (desflurane, USP) is a volatile liquid inhalation anesthetic minimally biotransformed in the liver in humans. Less than 0.02% of the desflurane absorbed can be recovered as urinary metabolites (compared to 0.2% for isoflurane). Eight healthy male volunteers first breathed 70% N2O/30% O2 for 30 minutes and then a mixture of desflurane 2.0%, isoflurane 0.4%, and halothane 0.2% for another 30 minutes. During this time, inspired and end-tidal concentrations (FI and FA) were measured. The FA/FI (washin) value at 30 minutes for desflurane was 0.91, compared to 1.00 for N2O, 0.74 for isoflurane, and 0.58 for halothane (see Figure 2). The washin rates for halothane and isoflurane were similar to literature values. The washin was faster for desflurane than for isoflurane and halothane at all time points. The FA/FAO (washout) value at 5 minutes was 0.12 for desflurane, 0.22 for isoflurane, and 0.25 for halothane (see Figure 3). The washout for desflurane was more rapid than that for isoflurane and halothane at all elimination time points. By 5 days, the FA/FAO for desflurane is 1/20th of that for halothane or isoflurane.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal carcinogenicity studies have not been performed with SUPRANE (desflurane, USP). In vitro and in vivo genotoxicity studies did not demonstrate mutagenicity or chromosomal damage by SUPRANE (desflurane, USP). Tests for genotoxicity included the Ames mutation assay, the metaphase analysis of human lymphocytes, and the mouse micronucleus assay.
Fertility was not affected after 1 MAC-Hour per day exposure (cumulative 63 and 14 MAC-Hours for males and females, respectively). At higher doses, parental toxicity (mortalities and reduced weight gain) was observed which could affect fertility.
14 CLINICAL STUDIES
The efficacy of SUPRANE (desflurane, USP) was evaluated in 1,843 patients including ambulatory (N=1,061), cardiovascular (N=277), geriatric (N=103), neurosurgical (N=40), and pediatric (N=235) patients. Clinical experience with these patients and with 1,087 control patients in these studies not receiving SUPRANE (desflurane, USP) is described below. Although SUPRANE (desflurane, USP) can be used in adults for the inhalation induction of anesthesia via mask, it produces a high incidence of respiratory irritation (coughing, breathholding, apnea, increased secretions, laryngospasm). Oxyhemoglobin saturation below 90% occurred in 6% of patients (from pooled data, N = 370 adults).
14.1 Ambulatory Surgery
SUPRANE (desflurane, USP) plus N2O was compared to isoflurane plus N2O in multicenter studies (21 sites) of 792 ASA physical status I, II, or III patients aged 18-76 years (median 32).
Induction
Anesthetic induction begun with thiopental and continued with SUPRANE (desflurane, USP) was associated with a 7% incidence of oxyhemoglobin saturation of 90% or less (from pooled data, N = 307) compared with 5% in patients in whom anesthesia was induced with thiopental and isoflurane (from pooled data, N = 152).
Maintenance & Recovery
SUPRANE (desflurane, USP) with or without N2O or other anesthetics was generally well tolerated. There were no differences between SUPRANE (desflurane, USP) and the other anesthetics studied in the times that patients were judged fit for discharge.
In one outpatient study, patients received a standardized anesthetic consisting of thiopental 4.2-4.4 mg/kg, fentanyl 3.5-4.0 µg/kg, vecuronium 0.05-0.07 mg/kg, and N2O 60% in oxygen with either desflurane 3% or isoflurane 0.6%. Emergence times were significantly different; but times to sit up and discharge were not different (see Table 5).
|
Recovery Profiles After Desflurane 3% in N2O 60% vs Isoflurane 0.6% in N2O 60% in Outpatients 16 Males, 22 Females, Ages 20-65 Mean ± SD |
||
| Isoflurane | Desflurane | |
| Number | 21 | 17 |
| Anesthetic time (min) | 127 ± 80 | 98 ± 55 |
| Recovery time to: | ||
| Follow commands (min) | 11.1 ± 7.9 | 6.5 ± 2.3 |
| Sit up (min) | 113 ± 27 | 95 ± 56 |
| Fit for discharge (min) | 231 ± 40 | 207 ± 54 |
14.2 Cardiovascular Surgery
SUPRANE (desflurane, USP) was compared to isoflurane, sufentanil or fentanyl for the anesthetic management of coronary artery bypass graft (CABG), abdominal aortic aneurysm, peripheral vascular and carotid endarterectomy surgery in 7 studies at 15 centers involving a total of 558 patients. In all patients except the desflurane vs. sufentanil study, the volatile anesthetics were supplemented with intravenous opioids, usually fentanyl. Blood pressure and heart rate were controlled by changes in concentration of the volatile anesthetics or opioids and cardiovascular drugs if necessary. Oxygen (100%) was the carrier gas in 253 of 277 desflurane cases (24 of 277 received N2O/O2).
|
Cardiovascular Patients by Agent and Type of Surgery 418 Males, 140 Females, Ages 27-87 (Median 64) |
||||||
|
Type of Surgery |
13 Centers | 1 Center | 1 Center | |||
| Isoflurane | Desflurane | Sufentanil | Desflurane | Fentanyl | Desflurane | |
| CABG | 58 | 57 | 100 | 100 | 25 | 25 |
| Abd Aorta | 29 | 25 | - | - | - | - |
| Periph Vasc | 24 | 24 | - | - | - | - |
| Carotid Art | 45 | 46 | - | - | - | - |
| Total | 156 | 152 | 100 | 100 | 25 | 25 |
No differences were found in cardiovascular outcome (death, myocardial infarction, ventricular tachycardia or fibrillation, heart failure) among desflurane and the other anesthetics.
Induction
SUPRANE (desflurane, USP) should not be used as the sole agent for anesthetic induction in patients with coronary artery disease or any patients where increases in heart rate or blood pressure are undesirable. In the desflurane vs. sufentanil study, anesthetic induction with desflurane without opioids was associated with new transient ischemia in 14 patients vs. 0 in the sufentanil group. In the desflurane group, mean heart rate, arterial pressure, and pulmonary blood pressure increased and stroke volume decreased in contrast to no change in the sufentanil group. Cardiovascular drugs were used frequently in both groups: especially esmolol in the desflurane group (56% vs. 0%) and phenylephrine in the sufentanil group (43% vs. 27%). When 10 µg/kg of fentanyl was used to supplement induction of anesthesia at one other center, continuous 2-lead ECG analysis showed a low incidence of myocardial ischemia and no difference between desflurane and isoflurane. If desflurane is to be used in patients with coronary artery disease, it should be used in combination with other medications for induction of anesthesia, preferably intravenous opioids and hypnotics.
Maintenance & Recovery
In studies where SUPRANE (desflurane, USP) or isoflurane anesthesia was supplemented with fentanyl, there were no differences in hemodynamic variables or the incidence of myocardial ischemia in the patients anesthetized with desflurane compared to those anesthetized with isoflurane.
During the precardiopulmonary bypass period, in the desflurane vs. sufentanil study where the desflurane patients received no intravenous opioid, more desflurane patients required cardiovascular adjuvants to control hemodynamics than the sufentanil patients. During this period, the incidence of ischemia detected by ECG or echocardiography was not statistically different between desflurane (18 of 99) and sufentanil (9 of 98) groups. However, the duration and severity of ECG-detected myocardial ischemia was significantly less in the desflurane group. The incidence of myocardial ischemia after cardiopulmonary bypass and in the ICU did not differ between groups.
14.3 Geriatric Surgery
SUPRANE (desflurane, USP) plus N2O was compared to isoflurane plus N2O in a multicenter study (6 sites) of 203 ASA physical status II or III elderly patients, aged 57-91 years (median 71).
Induction
Most patients were premedicated with fentanyl (mean 2 µg/kg), preoxygenated, and received thiopental (mean 4.3 mg/kg IV) or thiamylal (mean 4 mg/kg IV) followed by succinylcholine (mean 1.4 mg/kg IV) for intubation.
Maintenance & Recovery
Heart rate and arterial blood pressure remained within 20% of preinduction baseline values during administration of SUPRANE (desflurane, USP) 0.5-7.7% (average 3.6%) with 50-60% N2O. Induction, maintenance, and recovery cardiovascular measurements did not differ from those during isoflurane/N2O administration nor did the postoperative incidence of nausea and vomiting differ. The most common cardiovascular adverse event was hypotension occurring in 8% of the desflurane patients and 6% of the isoflurane patients.
14.4 Neurosurgery
SUPRANE (desflurane, USP) was studied in 38 patients aged 26-76 years (median 48 years), ASA physical status II or III undergoing neurosurgical procedures for intracranial lesions.
Induction
Induction consisted of standard neuroanesthetic techniques including hyperventilation and thiopental.
Maintenance
No change in cerebrospinal fluid pressure (CSFP) was observed in 8 patients who had intracranial tumors when the dose of SUPRANE (desflurane, USP) was 0.5 MAC in N2O 50%. In another study of 9 patients with intracranial tumors, 0.8 MAC desflurane/air/O2 did not increase CSFP above post induction baseline values. In a different study of 10 patients receiving 1.1 MAC desflurane/air/O2, CSFP increased 7 mm Hg (range 3-13 mm Hg increase, with final values of 11-26 mm Hg) above the pre-drug values.
All volatile anesthetics may increase intracranial pressure in patients with intracranial space occupying lesions. In such patients, SUPRANE (desflurane, USP) should be administered at 0.8 MAC or less, and in conjunction with a barbiturate induction and hyperventilation (hypocapnia) in the period before cranial decompression. Appropriate attention must be paid to maintain cerebral perfusion pressure. The use of a lower dose of SUPRANE (desflurane, USP) and the administration of a barbiturate and mannitol would be predicted to lessen the effect of desflurane on CSFP.
Under hypocapnic conditions (PaCO2 27 mm Hg) SUPRANE (desflurane, USP) 1 and 1.5 MAC did not increase cerebral blood flow (CBF) in 9 patients undergoing craniotomies. CBF reactivity to increasing PaCO2 from 27 to 35 mm Hg was also maintained at 1.25 MAC desflurane/air/O2.
14.5 Pediatric Surgery
In a clinical safety trial conducted in children aged 2 to 16 years (mean 7.4 years), following induction with another agent, SUPRANE (desflurane, USP) and isoflurane (in N2O/O2) were compared when delivered via face mask or laryngeal mask airway (LMA™ mask) for maintenance of anesthesia, after induction with intravenous propofol or inhaled sevoflurane, in order to assess the relative incidence of respiratory adverse events.
|
Maintenance in Nonintubated Pediatric Patients
All Respiratory Events |
||||
| All Ages (N=300) |
2-6 yr (N=150) |
7-11 yr (N=81) |
12-16 yr (N=69) |
|
| Any respiratory events | 39% | 42% | 33% | 39% |
| Airway obstruction | 4% | 5% | 4% | 3% |
| Breath-holding | 3% | 2% | 3% | 4% |
| Coughing | 26% | 33% | 19% | 22% |
| Laryngospasm | 13% | 16% | 7% | 13% |
| Secretion | 12% | 13% | 10% | 12% |
| Non-specific desaturation | 2% | 2% | 1% | 1% |
SUPRANE (desflurane, USP) was associated with higher rates (compared with isoflurane) of coughing, laryngospasm and secretions with an overall rate of respiratory events of 39%. Of the pediatric patients exposed to desflurane, 5% experienced severe laryngospasm (associated with significant desaturation; i.e. SpO2 of <90% for >15 seconds, or requiring succinylcholine), across all ages, 2-16 years old. Individual age group incidences of severe laryngospasm were 9% for 2-6 years old, 1% for 7-11 years old, and 1% for 12-16 years old. Removal of LMA™ mask under deep anesthesia (MAC range 0.6 – 2.3 with a mean of 1.12 MAC) was associated with a further increase in frequency of respiratory adverse events as compared to awake LMA™ mask removal or LMA™ mask removal under deep anesthesia with the comparator. The frequency and severity of non-respiratory adverse events were comparable between the two groups.
The incidence of respiratory events under these conditions was highest in children aged 2-6 years. Therefore, similar studies in children under the age of 2 years were not initiated.
16 HOW SUPPLIED/STORAGE AND HANDLING
SUPRANE (desflurane, USP) is available in an amber-colored glass bottle or an aluminum bottle containing 240 mL of desflurane as follows:
| NDC | Container | Unit(s) |
| 10019-641-60 | Amber-colored Glass | 1 |
| 10019-641-24 | 6 | |
| 10019-641-64 | Aluminum Bottle | 1 |
| 10019-641-34 | 6 |
16.1 Safety and Handling
Occupational Caution
There is no specific work exposure limit established for SUPRANE (desflurane, USP). However, the National Institute for Occupational Safety and Health Administration (NIOSH) recommends that no worker should be exposed at ceiling concentrations greater than 2 ppm of any halogenated anesthetic agent over a sampling period not to exceed one hour.
Principle routes of exposure include:
Skin contact – May cause skin irritation. In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Seek medical attention if irritation develops.
Eye contact – May cause eye irritation. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Seek medical attention if irritation develops.
Ingestion – No specific hazards other than therapeutic effects. Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, seek medical attention immediately.
Inhalation – If individuals smell vapors, or experience dizziness or headaches, they should be moved to an area with fresh air. Individuals could also experience the following: Cardiovascular effects: may include fluctuations in heart rate, changes in blood pressure, chest pain. Respiratory effects: may include shortness of breath, bronchospasms, laryngospasms, respiratory depression. Gastrointestinal effects: may include nausea, upset stomach, loss of appetite. Nervous System effects: may include ataxia, tremor, disturbance of speech, lethargy, headache, dizziness, blurred vision.
The predicted effects of acute overexposure by inhalation of SUPRANE (desflurane, USP) include headache, dizziness or (in extreme cases) unconsciousness [see Overdosage (10)].
There are no documented adverse effects of chronic exposure to halogenated anesthetic vapors (Waste Anesthetic Gases or WAGs) in the workplace. Although results of some epidemiological studies suggest a link between exposure to halogenated anesthetics and increased health problems (particularly spontaneous abortion), the relationship is not conclusive. Since exposure to WAGs is one possible factor in the findings for these studies, operating room personnel, and pregnant women in particular, should minimize exposure. Precautions include adequate general ventilation in the operating room, the use of a well-designed and well-maintained scavenging system; work practices to minimize leaks and spills while the anesthetic agent is in use, and routine equipment maintenance to minimize leaks.
Consistent with clinical data, concentrations would need to reach 2-3% in inspired air before individuals would likely experience dizziness or other physiologic effects.
16.2 Storage
Store at room temperature, 15°-30°C (59°-86°F). SUPRANE (desflurane, USP) has been demonstrated to be stable for the period defined by the expiration dating on the label. The bottle should be recapped after each use of SUPRANE (desflurane, USP).
17 PATIENT COUNSELING INFORMATION
Anesthesia providers need to obtain the following information from patients prior to administration of anesthesia:
- Medications they are taking, including herbal supplements
- Drug allergies, including allergic reactions to anesthetic agents (including hepatic sensitivity)
- Any history of severe reactions to prior administration of anesthetic
- If the patient or a member of the patient’s family has a history of malignant hyperthermia or if the patient has a history of Duchenne muscular dystrophy or other latent neuromuscular disease
Anesthesia providers should inform patients of the risks associated with SUPRANE (desflurane, USP):
- Post-operative nausea and vomiting and respiratory adverse effects including coughing.
- There is no information of the effects of SUPRANE (desflurane, USP) following anesthesia on the ability to operate an automobile or other heavy machinery. However, patients should be advised that the ability to perform such tasks may be impaired after receiving anesthetic agents.
************************************************************************
Baxter and Suprane are registered trademarks of Baxter International Inc.
LMA is a trademark of The Laryngeal Mask Company Limited.
Baxter logo
Manufactured for
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
************************************************************************
Revised 11/2013
07-19-72-483
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL

Suprane
NDC 10019-641-34
(desflurane, USP) 6 x 240 mL Bottles
Store at room temperature 15°-30°C (59°-86°F).
Exp 99 8888 Lot XXXXXXXXXX
(17) 889900 (10) XXXXXXXXXX
(01) 5 0310019 64134 3 (30) 0006
Mfd. for Baxter Healthcare Corporation, Deerfield, IL 60015 USA
0000
07-06-71-114
SUPRANEdesflurane LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||