Stona Cough
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients (in 10 mL)
Dextromethorphan hydrobromide 30 mg
Guaifenesin 200 mg
Purpose
Purpose
Dextromethorphan hydrobromide Cough suppressant
Guaifenesin Expectorant
Uses
Uses
For the temporary relief of cough due to the common cold. Helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive.
WARNINGS
Enter section text here
Do not use this product
■if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
■for a persistent or chronic cough such as occurs with smoking, asthma, or emphysema.
■if cough is accompanied by excessive phlegm (mucos) unless directed by a doctor.
A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor.
Keep our of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Directions
■Adults and children 12 years of age and over: Take 10mL every 6 – 8 hours, not to exceed 30mL in 24 hours, or as directed by a doctor.
■Children under 12 years: Ask a doctor.
Inactive ingredients
alcohol, beta-cyclodextrin, butylparaben, calcium chloride, caramel, citric acid, flavor, glycyrrhiza (licorice) extract, propylparaben, sodium benzoate, sodium saccharin, sugar, water.
stonacoughsyrPDP.jpg
stonacoughsyrcartA.jpg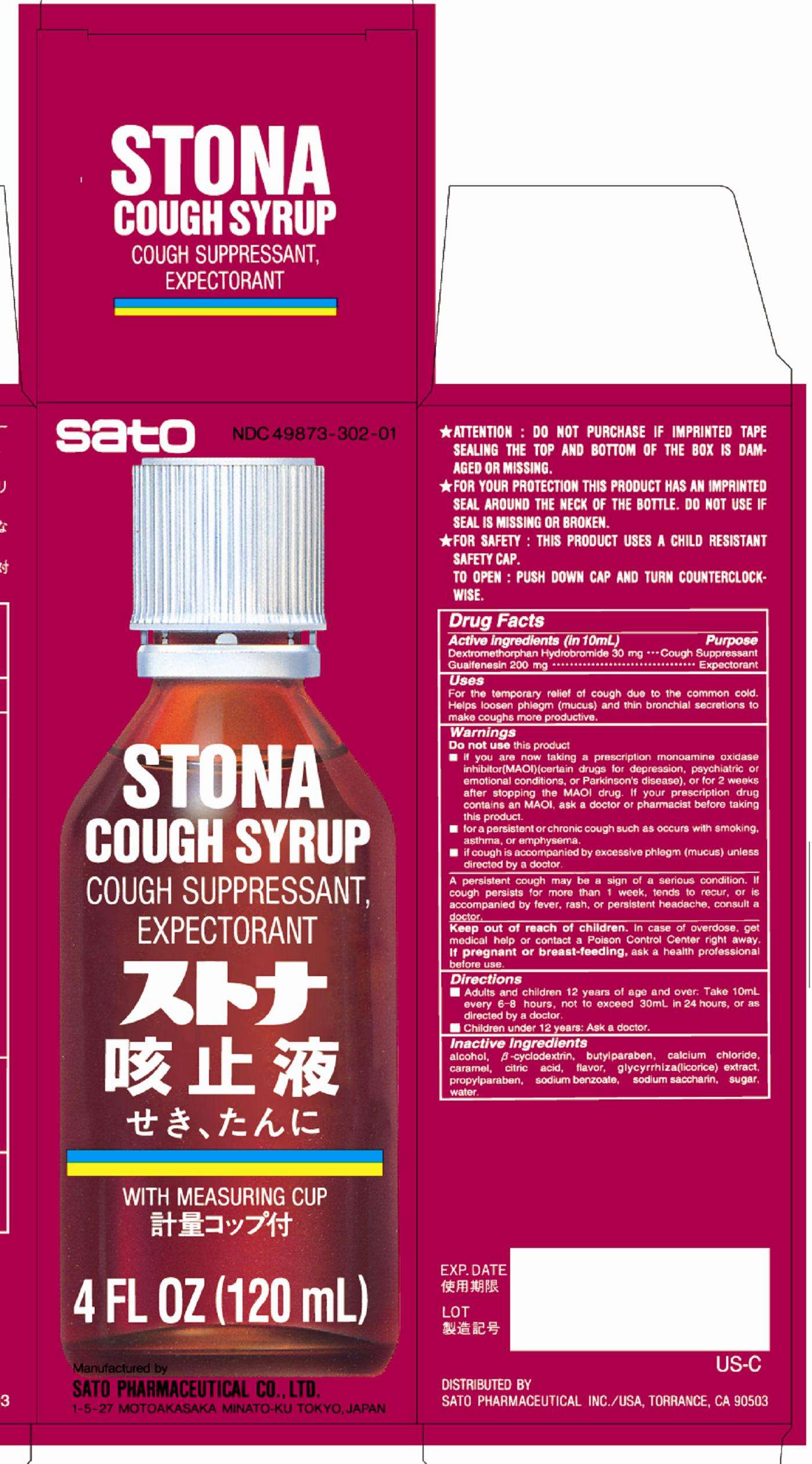
stonacoughsyrcartB.jpg
Stona Coughdextromethorphan hydrobromide, guaifenesin SYRUP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||