Soliris
HIGHLIGHTS OF PRESCRIBING INFORMATION BOXED WARNING WARNING: SERIOUS MENINGOCOCCAL INFECTIONS See full prescribing information for complete boxed warning Life-threatening and fatal meningococcal infections have occurred in patients treated with Soliris and may become rapidly life-threatening or fatal if not recognized and treated early (5.1). Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccination in patients with complement deficiencies (5.1). Immunize patients with a meningococcal vaccine at least 2 weeks prior to administering the first dose of Soliris, unless the risks of delaying Soliris therapy outweigh the risks of developing a meningococcal infection. (See Serious Meningococcal Infections (5.1) for additional guidance on the management of the risk of meningococcal infection.) Monitor patients for early signs of meningococcal infections, and evaluate immediately if infection is suspected. Soliris is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the Soliris REMS, prescribers must enroll in the program (5.2). RECENT MAJOR CHANGESDosage and Administration (2.4) 4/2014INDICATIONS AND USAGESoliris is a complement inhibitor indicated for: The treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis (1.1). The treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy (1.2). Limitation of Use Soliris is not indicated for the treatment of patients with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS).DOSAGE AND ADMINISTRATIONOnly administer as an intravenous infusion PNH Dosage Regimen: (2.1)aHUS Dosage Regimen: (2.2)DOSAGE FORMS AND STRENGTHS300 mg single-use vials each containing 30 mL of 10 mg/mL sterile, preservative-free solution (3).CONTRAINDICATIONSSoliris is contraindicated in: Patients with unresolved serious Neisseria meningitidis infection (4). Patients who are not currently vaccinated against Neisseria meningitidis, unless the risks of delaying Soliris treatment outweigh the risks of developing a meningococcal infection (5.1). WARNINGS AND PRECAUTIONS Discontinue Soliris in patients who are being treated for serious meningococcal infections. Use caution when administering Soliris to patients with any other systemic infection (5.2). Side EffectsThe most frequently reported adverse reactions in the PNH randomized trial (≥10% overall and greater than placebo) are: headache, nasopharyngitis, back pain, and nausea (6.1).The most frequently reported adverse reactions in aHUS single arm prospective trials (≥20%) are: headache, diarrhea, hypertension, upper respiratory infection, abdominal pain, vomiting, nasopharyngitis, anemia, cough, peripheral edema, nausea, urinary tract infections, pyrexia (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Alexion Pharmaceuticals, Inc. at 1-888-SOLIRIS (1-888-765-4747) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .USE IN SPECIFIC POPULATIONSPregnancy: Based on animal data, Soliris may cause fetal harm (8.1).Nursing Mothers: Caution should be exercised when administered to a nursing woman (8.3).Pediatric Use: PNH: safety and effectiveness not established. aHUS: safety and effectiveness similar to adult patients (8.4).
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 SOLIRIS INDICATIONS AND USAGE
- 2 SOLIRIS DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 SOLIRIS CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 SOLIRIS ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 SOLIRIS DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
Life-threatening and fatal meningococcal infections have occurred in patients treated with Soliris. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early [see Warnings and Precautions (5.1)].
- Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccination in patients with complement deficiencies.
- Immunize patients with a meningococcal vaccine at least 2 weeks prior to administering the first dose of Soliris, unless the risks of delaying Soliris therapy outweigh the risk of developing a meningococcal infection [See Warnings and Precautions (5.1) for additional guidance on the management of the risk of meningococcal infection].
- Monitor patients for early signs of meningococcal infections and evaluate immediately if infection is suspected.
Soliris is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the Soliris REMS, prescribers must enroll in the program [see Warnings and Precautions (5.2) ]. Enrollment in the Soliris REMS program and additional information are available by telephone: 1-888-SOLIRIS (1-888-765-4747).
1 INDICATIONS AND USAGE
1.1 Paroxysmal Nocturnal Hemoglobinuria (PNH)
Soliris is indicated for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis.
1.2 Atypical Hemolytic Uremic Syndrome (aHUS)
Soliris is indicated for the treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy.
Limitation of Use
Soliris is not indicated for the treatment of patients with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS).
2 DOSAGE AND ADMINISTRATION
Healthcare professionals who prescribe Soliris must enroll in the Soliris REMS. [see Warnings and Precautions (5.2)].
Vaccinate patients according to current ACIP guidelines to reduce the risk of serious infection. [see Warnings and Precautions (5.1) and (5.2)].
Only administer as an intravenous infusion.
2.1 Recommended Dosage Regimen - PNH
Soliris therapy consists of:
- 600 mg weekly for the first 4 weeks, followed by
- 900 mg for the fifth dose 1 week later, then
- 900 mg every 2 weeks thereafter.
Soliris should be administered at the recommended dosage regimen time points, or within two days of these time points [see Warnings and Precautions (5.6)].
2.2 Recommended Dosage Regimen - aHUS
For patients 18 years of age and older, Soliris therapy consists of:
- 900 mg weekly for the first 4 weeks, followed by
- 1200 mg for the fifth dose 1 week later, then
- 1200 mg every 2 weeks thereafter.
For patients less than 18 years of age, administer Soliris based upon body weight, according to the following schedule (Table 1):
| Patient Body Weight | Induction | Maintenance |
| 40 kg and over | 900 mg weekly x 4 doses |
1200 mg at week 5; then 1200 mg every 2 weeks |
| 30 kg to less than 40 kg | 600 mg weekly x 2 doses |
900 mg at week 3; then 900 mg every 2 weeks |
| 20 kg to less than 30 kg | 600 mg weekly x 2 doses |
600 mg at week 3; then 600 mg every 2 weeks |
| 10 kg to less than 20 kg | 600 mg weekly x 1 dose |
300 mg at week 2; then 300 mg every 2 weeks |
| 5 kg to less than 10 kg | 300 mg weekly x 1 dose |
300 mg at week 2; then 300 mg every 3 weeks |
Soliris should be administered at the recommended dosage regimen time points, or within two days of these time points.
Supplemental dosing of Soliris is required in the setting of concomitant support with PE/PI (plasmapheresis or plasma exchange; or fresh frozen plasma infusion) (Table 2).
| Type of Intervention | Most Recent Soliris Dose | Supplemental Soliris Dose With Each PE/PI Intervention | Timing of Supplemental Soliris Dose |
| Plasmapheresis or plasma exchange | 300 mg | 300 mg per each plasmapheresis or plasma exchange session | Within 60 minutes after each plasmapheresis or plasma exchange |
| 600 mg or more | 600 mg per each plasmapheresis or plasma exchange session | ||
| Fresh frozen plasma infusion | 300 mg or more | 300 mg per infusion of fresh frozen plasma | 60 minutes prior to each infusion of fresh frozen plasma |
2.3 Preparation and Administration
Soliris must be diluted to a final admixture concentration of 5 mg/mL using the following steps:
- Withdraw the required amount of Soliris from the vial into a sterile syringe.
- Transfer the recommended dose to an infusion bag.
- Dilute Soliris to a final concentration of 5 mg/mL by adding the appropriate amount (equal volume of diluent to drug volume) of 0.9% Sodium Chloride Injection, USP; 0.45% Sodium Chloride Injection, USP; 5% Dextrose in Water Injection, USP; or Ringer's Injection, USP to the infusion bag.
The final admixed Soliris 5 mg/mL infusion volume is 60 mL for 300 mg doses, 120 mL for 600 mg doses, 180 mL for 900 mg doses or 240 mL for 1200 mg doses (Table 3).
| Soliris Dose | Diluent Volume | Final Volume |
| 300 mg | 30 mL | 60 mL |
| 600 mg | 60 mL | 120 mL |
| 900 mg | 90 mL | 180 mL |
| 1200 mg | 120 mL | 240 mL |
Gently invert the infusion bag containing the diluted Soliris solution to ensure thorough mixing of the product and diluent. Discard any unused portion left in a vial, as the product contains no preservatives.
Prior to administration, the admixture should be allowed to adjust to room temperature [18-25° C, 64-77° F]. The admixture must not be heated in a microwave or with any heat source other than ambient air temperature. The Soliris admixture should be inspected visually for particulate matter and discoloration prior to administration.
2.4 Administration
Do Not Administer As An Intravenous Push or Bolus Injection
The Soliris admixture should be administered by intravenous infusion over 35 minutes in adults and 1 to 4 hours in pediatric patients via gravity feed, a syringe-type pump, or an infusion pump. Admixed solutions of Soliris are stable for 24 hours at 2-8° C (36-46° F) and at room temperature.
If an adverse reaction occurs during the administration of Soliris, the infusion may be slowed or stopped at the discretion of the physician. If the infusion is slowed, the total infusion time should not exceed two hours in adults. Monitor the patient for at least one hour following completion of the infusion for signs or symptoms of an infusion reaction.
3 DOSAGE FORMS AND STRENGTHS
Soliris is supplied as 300 mg single-use vials each containing 30 mL of 10 mg/mL sterile, preservative-free eculizumab solution.
4 CONTRAINDICATIONS
Soliris is contraindicated in:
- Patients with unresolved serious Neisseria meningitidis infection.
- Patients who are not currently vaccinated against Neisseria meningitidis, unless the risks of delaying Soliris treatment outweigh the risks of developing a meningococcal infection [see Warnings and Precautions (5.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Serious Meningococcal Infections
The use of Soliris increases a patient's susceptibility to serious meningococcal infections (septicemia and/or meningitis). Life-threatening and fatal meningococcal infections have occurred in patients treated with Soliris.
Administer a polyvalent meningococcal vaccine according to the most current Advisory Committee on Immunization Practices (ACIP) recommendations for patients with complement deficiencies. Revaccinate patients in accordance with ACIP recommendations, considering the duration of Soliris therapy.
Immunize patients without a history of meningococcal vaccination at least 2 weeks prior to receiving the first dose of Soliris. If urgent Soliris therapy is indicated in an unvaccinated patient, administer the meningococcal vaccine as soon as possible. In prospective clinical studies, 75/100 patients with aHUS were treated with Soliris less than 2 weeks after meningococcal vaccination and 64 of these 75 patients received antibiotics for prophylaxis of meningococcal infection until at least 2 weeks after meningococcal vaccination. The benefits and risks of antibiotic prophylaxis for prevention of meningococcal infections in patients receiving Soliris have not been established.
Vaccination reduces, but does not eliminate, the risk of meningococcal infections. In clinical studies, 2 out of 196 PNH patients developed serious meningococcal infections while receiving treatment with Soliris; both had been vaccinated [see Adverse Reactions (6.1) ]. In clinical studies among non-PNH patients, meningococcal meningitis occurred in one unvaccinated patient. In addition, 3 out of 130 previously vaccinated patients with aHUS developed meningococcal infections while receiving treatment with Soliris [see Adverse Reactions (6.1) ].
Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if an infection is suspected. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. Discontinue Soliris in patients who are undergoing treatment for serious meningococcal infections.
5.2 Soliris REMS
Because of the risk of meningococcal infections, Soliris is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the Soliris REMS, prescribers must enroll in the program.
Prescribers must counsel patients about the risk of meningococcal infection, provide the patients with the REMS educational materials, and ensure patients are vaccinated with a meningococcal vaccine.
Enrollment in the Soliris REMS program and additional information are available by telephone: 1-888-SOLIRIS (1-888-765-4747).
5.3 Other Infections
Soliris blocks terminal complement activation; therefore patients may have increased susceptibility to infections, especially with encapsulated bacteria. Additionally, Aspergillus infections have occurred in immunocompromised and neutropenic patients. Children treated with Soliris may be at increased risk of developing serious infections due to Streptococcus pneumoniae and Haemophilus influenza type b (Hib). Administer vaccinations for the prevention of Streptococcus pneumoniae and Haemophilus influenza type b (Hib) infections according to ACIP guidelines. Use caution when administering Soliris to patients with any systemic infection.
5.4 Monitoring Disease Manifestations After Soliris Discontinuation
Treatment Discontinuation for PNH
Monitor patients after discontinuing Soliris for at least 8 weeks to detect hemolysis.
Treatment Discontinuation for aHUS
After discontinuing Soliris, monitor patients with aHUS for signs and symptoms of thrombotic microangiopathy (TMA) complications for at least 12 weeks. In aHUS clinical trials, 18 patients (5 in the prospective studies) discontinued Soliris treatment. TMA complications occurred following a missed dose in 5 patients, and Soliris was reinitiated in 4 of these 5 patients.
Clinical signs and symptoms of TMA include changes in mental status, seizures, angina, dyspnea, or thrombosis. In addition, the following changes in laboratory parameters may identify a TMA complication: occurrence of two, or repeated measurement of any one of the following: a decrease in platelet count by 25% or more compared to baseline or the peak platelet count during Soliris treatment; an increase in serum creatinine by 25% or more compared to baseline or nadir during Soliris treatment; or, an increase in serum LDH by 25% or more over baseline or nadir during Soliris treatment.
If TMA complications occur after Soliris discontinuation, consider reinstitution of Soliris treatment, plasma therapy [plasmapheresis, plasma exchange, or fresh frozen plasma infusion (PE/PI)], or appropriate organ-specific supportive measures.
5.5 Thrombosis Prevention and Management
The effect of withdrawal of anticoagulant therapy during Soliris treatment has not been established. Therefore, treatment with Soliris should not alter anticoagulant management.
5.6 Infusion Reactions
As with all protein products, administration of Soliris may result in infusion reactions, including anaphylaxis or other hypersensitivity reactions. In clinical trials, no patients experienced an infusion reaction which required discontinuation of Soliris. Interrupt Soliris infusion and institute appropriate supportive measures if signs of cardiovascular instability or respiratory compromise occur.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Serious Meningococcal Infections [See Warnings and Precautions (5.1)]
- Other Infections [See Warnings and Precautions (5.3)]
- Monitoring Disease Manifestations After Soliris Discontinuation [See Warnings and Precautions (5.4)]
- Thrombosis Prevention and Management [See Warnings and Precautions (5.5)]
- Infusion Reactions [See Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Meningococcal infections are the most important adverse reactions experienced by patients receiving Soliris. In PNH clinical studies, two patients experienced meningococcal sepsis. Both patients had previously received a meningococcal vaccine. In clinical studies among patients without PNH, meningococcal meningitis occurred in one unvaccinated patient. Meningococcal sepsis occurred in one previously vaccinated patient enrolled in the retrospective aHUS study during the post-study follow-up period [see Warnings and Precautions (5.1)].
PNH
The data described below reflect exposure to Soliris in 196 adult patients with PNH, age 18-85, of whom 55% were female. All had signs or symptoms of intravascular hemolysis. Soliris was studied in a placebo-controlled clinical study (in which 43 patients received Soliris and 44, placebo); a single arm clinical study and a long term extension study. 182 patients were exposed for greater than one year. All patients received the recommended Soliris dose regimen.
Table 4 summarizes the adverse reactions that occurred at a numerically higher rate in the Soliris group than the placebo group and at a rate of 5% or more among patients treated with Soliris.
| Reaction | Soliris | Placebo |
| N = 43 | N = 44 | |
| N (%) | N (%) | |
| Headache | 19 (44) | 12 (27) |
| Nasopharyngitis | 10 (23) | 8 (18) |
| Back pain | 8 (19) | 4 (9) |
| Nausea | 7 (16) | 5 (11) |
| Fatigue | 5 (12) | 1 (2) |
| Cough | 5 (12) | 4 (9) |
| Herpes simplex infections | 3 (7) | 0 |
| Sinusitis | 3 (7) | 0 |
| Respiratory tract infection | 3 (7) | 1 (2) |
| Constipation | 3 (7) | 2 (5) |
| Myalgia | 3 (7) | 1 (2) |
| Pain in extremity | 3 (7) | 1 (2) |
| Influenza-like illness | 2 (5) | 1 (2) |
In the placebo-controlled clinical study, serious adverse reactions occurred among 4 (9%) patients receiving Soliris and 9 (21%) patients receiving placebo. The serious reactions included infections and progression of PNH. No deaths occurred in the study and no patients receiving Soliris experienced a thrombotic event; one thrombotic event occurred in a patient receiving placebo.
Among 193 patients with PNH treated with Soliris in the single arm, clinical study or the follow-up study, the adverse reactions were similar to those reported in the placebo-controlled clinical study. Serious adverse reactions occurred among 16% of the patients in these studies. The most common serious adverse reactions were: viral infection (2%), headache (2%), anemia (2%), and pyrexia (2%).
aHUS
The safety of Soliris therapy in patients with aHUS was evaluated in four prospective, single-arm studies, three in adult and adolescent patients (aHUS Studies 1, 2, and 4), one in pediatric and adolescent patients (aHUS Study 5) and one retrospective study (aHUS Study 3).
The data described below were derived from 78 adult and adolescent patients with aHUS enrolled in aHUS Study 1, aHUS Study 2, and aHUS Study 4. All patients received the recommended dosage of Soliris. Median exposure was 67 weeks (range: 2-145 weeks). Table 5 summarizes all adverse events reported in at least 10% of patients in aHUS Studies 1, 2, and 4 combined.
| MedDRA ver. 15.1 | Number (%) of Patients | |||
|
Study 1 (n=17) |
Study 2 (n=20) |
Study 4 (n=41) |
Total (n=78) |
|
| Vascular Disorders | ||||
| Hypertensiona | 10 (59) | 9 (45) | 7 (17) | 26 (33) |
| Hypotension | 2 (12) | 4 (20) | 7 (17) | 13 (17) |
| Infections and Infestations | ||||
| Bronchitis | 3 (18) | 2 (10) | 4 (10) | 9 (12) |
| Nasopharyngitis | 3 (18) | 11 (55) | 7 (17) | 21 (27) |
| Gastroenteritis | 3 (18) | 4 (20) | 2 (5) | 9 (12) |
| Upper respiratory tract infection | 5 (29) | 8 (40) | 2 (5) | 15 (19) |
| Urinary tract infection | 6 (35.3) | 3 (15) | 8 (20) | 17 (22) |
| Gastrointestinal Disorders | ||||
| Diarrhea | 8 (47) | 8 (40) | 12 (32) | 29 (37) |
| Vomiting | 8 (47) | 9 (45) | 6 (15) | 23 (30) |
| Nausea | 5 (29) | 8 (40) | 5 (12) | 18 (23) |
| Abdominal pain | 3 (18) | 6 (30) | 6 (15) | 15 (19) |
| Nervous System Disorders | ||||
| Headache | 7 (41) | 10 (50) | 15 (37) | 32 (41) |
| Blood and Lymphatic System Disorders | ||||
| Anemia | 6 (35) | 7 (35) | 7 (17) | 20 (26) |
| Leukopenia | 4 (24) | 3 (15) | 5 (12) | 12 (15) |
| Psychiatric Disorders | ||||
| Insomnia | 4 (24) | 2 (10) | 5 (12) | 11 (14) |
| Renal and Urinary Disorders | ||||
| Renal Impairment | 5 (29) | 3 (15) | 6 (15) | 14 (18) |
| Proteinuria | 2 (12) | 1 (5) | 5 (12) | 8 (10) |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Cough | 4 (24) | 6 (30) | 8 (20) | 18 (23) |
| General Disorders and Administration Site Conditions | ||||
| Fatigue | 3 (18) | 4 (20) | 3 (7) | 10 (13) |
| Peripheral edema | 5 (29) | 4 (20) | 9 (22) | 18 (23) |
| Pyrexia | 4 (24) | 5 (25) | 7 (17) | 16 (21) |
| Asthenia | 3 (18) | 4 (20) | 6 (15) | 13 (17) |
| Eye Disorder | 5 (29) | 2 (10) | 8 (20) | 15 (19) |
| Metabolism and Nutrition Disorders | ||||
| Hypokalaemia | 3 (18) | 2 (10) | 4 (10) | 9 (12) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 (6) | 6 (30) | 1 (20) | 8 (10) |
| Skin and Subcutaneous Tissue Disorders | ||||
| Rash | 2 (12) | 3 (15) | 6 (15) | 11 (14) |
| Pruritis | 1 (6) | 3 (15) | 4 (10) | 8 (10) |
| Musculoskeletal and Connective Tissue Disorders | ||||
| Arthralgia | 1 (6) | 2 (10) | 7 (17) | 10 (13) |
| Back pain | 3 (18) | 3 (15) | 2 (5) | 8 (10) |
a includes the preferred terms hypertension, accelerated hypertension, and malignant hypertension.
In aHUS Studies 1, 2, and 4 combined, 60% (47/78) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were infections (24%), hypertension (5%), chronic renal failure (5%), and renal impairment (5%). Five patients discontinued Soliris due to adverse events; three due to worsening renal function, one due to new diagnosis of Systemic Lupus Erythematosus, and one due to meningoccal meningitis.
aHUS Study 5 included 22 pediatric and adolescent patients, of which 18 patients were less than 12 years of age. All patients received the recommended dosage of Soliris. Median exposure was 44 weeks (range: 1 dose-87 weeks).
Table 6 summarizes all adverse events reported in at least 10% of patients enrolled in aHUS Study 5.
| MedDRA ver. 15.1 | ||
| 1 month to <12 yrs | Total | |
| (n=18) | (n=22) | |
| Eye Disorders | 3 (17) | 3 (14) |
| Gastrointestinal Disorders | ||
| Abdominal pain | 6 (33) | 7 (32) |
| Diarrhoea | 5 (28) | 7 (32) |
| Vomiting | 4 (22) | 6 (27) |
| Dyspepsia | 0 | 3 (14) |
| General Disorders and Administration Site Conditions | ||
| Pyrexia | 9 (50) | 11 (50) |
| Infections and Infestations | ||
| Upper respiratory tract infection | 5 (28) | 7 (32) |
| Nasopharyngitis | 3 (17) | 6 (27) |
| Rhinitis | 4 (22) | 4 (18) |
| Urinary Tract infection | 3 (17) | 4 (18) |
| Catheter site infection | 3 (17) | 3 (14) |
| Musculoskeletal and Connective Tissue Disorders | ||
| Muscle spasms | 2 (11) | 3 (14) |
| Nervous System Disorders | ||
| Headache | 3 (17) | 4 (18) |
| Renal and Urinary Disorders | 3 (17) | 4 (18) |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough | 7 (39) | 8 (36) |
| Oropharyngeal pain | 1 (6) | 3 (14) |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 4 (22) | 4 (18) |
| Vascular Disorders | ||
| Hypertension | 4 (22) | 4 (18) |
In aHUS Study 5, 59% (13/22) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were hypertension (9%), viral gastroenteritis (9%), pyrexia (9%), and upper respiratory infection (9%). One patient discontinued Soliris due to an adverse event (severe agitation).
Analysis of retrospectively collected adverse event data from pediatric and adult patients enrolled in aHUS Study 3 (N=30) revealed a safety profile that was similar to that which was observed in the two prospective studies. aHUS Study 3 included 19 pediatric patients less than 18 years of age. Overall, the safety of Soliris in pediatric patients with aHUS enrolled in Study 3 appeared similar to that observed in adult patients. The most common (≥15%) adverse events occurring in pediatric patients are presented in Table 7.
| MedDRA ver. 11.0 | Number (%) of Patients | |||
| < 2 yrs | 2 to < 12 yrs | 12 to <18 yrs | Total | |
| (n=5) | (n=10) | (n=4) | (n=19) | |
| General Disorders and Administration Site Conditions | ||||
| Pyrexia | 4 (80) | 4 (40) | 1 (25) | 9 (47) |
| Gastrointestinal Disorders | ||||
| Diarrhea | 1 (20) | 4 (40) | 1 (25) | 6 (32) |
| Vomiting | 2 (40) | 1 (10) | 1 (25) | 4 (21) |
| Infections and Infestations | ||||
| Upper respiratory tract infectiona | 2 (40) | 3 (30) | 1 (25) | 6 (32) |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Cough | 3 (60) | 2 (20) | 0 (0) | 5 (26) |
| Nasal congestion | 2 (40) | 2 (20) | 0 (0) | 4 (21) |
| Cardiac Disorders | ||||
| Tachycardia | 2 (40) | 2 (20) | 0 (0) | 4 (21) |
a. includes the preferred terms upper respiratory tract infection and nasopharyngitis.
6.2 Immunogenicity
As with all proteins, there is a potential for immunogenicity with eculizumab. The immunogenicity of Soliris has been evaluated using two different immunoassays for the detection of anti-eculizumab antibodies: a direct enzyme-linked immunosorbent assay (ELISA) using the Fab fragment of eculizumab as target was used for the PNH indication; and an electro-chemiluminescence (ECL) bridging assay using the eculizumab whole molecule as target was used for the aHUS indication, as well as for additional patients with PNH. In the PNH population, antibodies to Soliris were detected in 3/196 (2%) patients with PNH treated with Soliris using the ELISA assay and in 5/161 (3%) patients treated with Soliris using the ECL assay. In patients with aHUS treated with Soliris, antibodies to Soliris were detected in 3/100 (3%) using the ECL assay. An ECL based neutralizing HAHA assay with a low sensitivity of 2 mcg/mL was performed to detect neutralizing antibodies for the 3 patients with aHUS and also for the 5 patients with PNH with positive samples using the ECL assay. 2/161 patients in the PNH group (1.2%) and 1/100 patients in the aHUS group (1%) had low positive values for neutralizing antibodies. No apparent correlation of antibody development to clinical response was observed in either indication. The immunogenicity data reflect the percentage of patients whose test results were considered positive for antibodies to Soliris in an ELISA-based assay and/or an ECL-based assay and are highly dependent on the sensitivity and specificity of the assay used. Additionally, the observed incidence of antibody positivity in the assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to Soliris with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Soliris. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Soliris exposure.
Cases of serious or fatal meningococcal infections have been reported.
7 DRUG INTERACTIONS
Drug interaction studies have not been performed with Soliris.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C:
Risk Summary
There are no adequate and well-controlled studies of Soliris in pregnant women. Soliris, a recombinant IgG molecule (humanized anti-C5 antibody), is expected to cross the placenta. Animal studies using a mouse analogue of the Soliris molecule (murine anti-C5 antibody) showed increased rates of developmental abnormalities and an increased rate of dead and moribund offspring at doses 2-8 times the human dose. Soliris should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
Animal reproduction studies were conducted in mice using doses of a murine anti-C5 antibody that approximated 2-4 times (low dose) and 4-8 times (high dose) the recommended human Soliris dose, based on a body weight comparison. When animal exposure to the antibody occurred in the time period from before mating until early gestation, no decrease in fertility or reproductive performance was observed. When maternal exposure to the antibody occurred during organogenesis, two cases of retinal dysplasia and one case of umbilical hernia were observed among 230 offspring born to mothers exposed to the higher antibody dose; however, the exposure did not increase fetal loss or neonatal death. When maternal exposure to the antibody occurred in the time period from implantation through weaning, a higher number of male offspring became moribund or died (1/25 controls, 2/25 low dose group, 5/25 high dose group). Surviving offspring had normal development and reproductive function.
8.3 Nursing Mothers
It is not known whether Soliris is excreted into human milk. IgG is excreted in human milk, so it is expected that Soliris will be present in human milk. However, published data suggest that antibodies in human milk do not enter the neonatal and infant circulation in substantial amounts. Caution should be exercised when Soliris is administered to a nursing woman. The unknown risks to the infant from gastrointestinal or limited systemic exposure to Soliris should be weighed against the known benefits of human milk feeding.
8.4 Pediatric Use
The safety and effectiveness of Soliris for the treatment of PNH in pediatric patients below the age of 18 years have not been established.
Four clinical studies assessing the safety and effectiveness of Soliris for the treatment of aHUS included a total of 47 pediatric patients (ages 2 months to 17 years). The safety and effectiveness of Soliris for the treatment of aHUS appear similar in pediatric and adult patients [see Dosage and Administration (2.2), Adverse Reactions (6.1), and Clinical Studies (14.2)].
Administer vaccinations for the prevention of infection due to Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to ACIP guidelines [see Warnings and Precautions (5.1, 5.2)].
8.5 Geriatric Use
Nineteen patients 65 years of age or older (15 with PNH and 4 with aHUS) were treated with Soliris. Although there were no apparent age-related differences observed in these studies, the number of patients aged 65 and over is not sufficient to determine whether they respond differently from younger patients.
10 OVERDOSAGE
No cases of Soliris overdose have been reported during clinical studies.
11 DESCRIPTION
Soliris, a complement inhibitor, is a formulation of eculizumab which is a recombinant humanized monoclonal IgG2/4κ antibody produced by murine myeloma cell culture and purified by standard bioprocess technology. Eculizumab contains human constant regions from human IgG2 sequences and human IgG4 sequences and murine complementarity-determining regions grafted onto the human framework light- and heavy-chain variable regions. Eculizumab is composed of two 448 amino acid heavy chains and two 214 amino acid light chains and has a molecular weight of approximately 148 kDa.
Soliris is a sterile, clear, colorless, preservative-free 10 mg/mL solution for intravenous infusion and is supplied in 30 mL single-use vials. The product is formulated at pH 7 and each vial contains 300 mg of eculizumab, 13.8 mg sodium phosphate monobasic, 53.4 mg sodium phosphate dibasic, 263.1 mg sodium chloride, 6.6 mg polysorbate 80 (vegetable origin) and Water for Injection, USP.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Eculizumab, the active ingredient in Soliris, is a monoclonal antibody that specifically binds to the complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a and C5b and preventing the generation of the terminal complement complex C5b-9. Soliris inhibits terminal complement mediated intravascular hemolysis in PNH patients and complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS.
A genetic mutation in patients with PNH leads to the generation of populations of abnormal RBCs (known as PNH cells) that are deficient in terminal complement inhibitors, rendering PNH RBCs sensitive to persistent terminal complement-mediated destruction. The destruction and loss of these PNH cells (intravascular hemolysis) results in low RBC counts (anemia), and also fatigue, difficulty in functioning, pain, dark urine, shortness of breath, and blood clots.
In aHUS, impairment in the regulation of complement activity leads to uncontrolled terminal complement activation, resulting in platelet activation, endothelial cell damage and thrombotic microangiopathy.
12.2 Pharmacodynamics
In the PNH placebo-controlled clinical study, Soliris when administered as recommended reduced hemolysis as shown by the reduction of serum LDH levels from 2200 ± 1034 U/L (mean ± SD) at baseline to 700 ± 388 U/L by week one and maintained the effect through the end of the study at week 26 (327 ± 433 U/L). In the single arm clinical study, Soliris maintained this effect through 52 weeks [see Clinical Studies (14)].
12.3 Pharmacokinetics
A population PK analysis with a standard 1-compartmental model was conducted on the multiple dose PK data from 40 PNH patients receiving the recommended Soliris regimen [see Dosage and Administration (2.1) ]. In this model, the clearance of Soliris for a typical PNH patient weighing 70 kg was 22 mL/hr and the volume of distribution was 7.7 L. The half-life was 272 ± 82 hrs (mean ± SD). The mean observed peak and trough serum concentrations of Soliris by week 26 were 194 ± 76 mcg/mL and 97 ± 60 mcg/mL, respectively.
A second population PK analysis with a standard 1 compartmental model was conducted on the multiple dose PK data from 57 aHUS patients receiving the recommended Soliris regimen in studies 1, 2 and 3. In this model, the clearance of Soliris for a typical aHUS patient weighing 70 kg was 14.6 mL/hr and the volume of distribution was 6.14 L. The elimination half-life was 291 h (approximately 12.1 days).
The clearance and half-life of eculizumab were also evaluated during plasma exchange interventions. Plasma exchange increased the clearance of eculizumab to 3660 mL/hr and reduced the half-life to 1.26 hours. Supplemental dosing is recommended when Soliris is administered to aHUS patients receiving plasma infusion or exchange [see Dosage and Administration (2.2) ].
Dedicated studies have not been conducted to evaluate the PK of Soliris in special patient populations identified by gender, race, age (geriatric), or the presence of renal or hepatic impairment. Pediatric and adolescent patients (less than 18 years of age) and patients with renal impairment were included in the aHUS clinical studies [see Clinical Studies (14) ]. Population PK analysis showed age, gender, race, and renal function do not influence the PK of eculizumab.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal carcinogenicity studies of eculizumab have not been conducted.
Genotoxicity studies have not been conducted with eculizumab.
Effects of eculizumab upon fertility have not been studied in animals. Intravenous injections of male and female mice with a murine anti-C5 antibody at up to 4-8 times the equivalent of the clinical dose of Soliris had no adverse effects on mating or fertility.
14 CLINICAL STUDIES
14.1 PNH
The safety and efficacy of Soliris in PNH patients with hemolysis were assessed in a randomized, double-blind, placebo-controlled 26 week study (Study 1); PNH patients were also treated with Soliris in a single arm 52 week study (Study 2); and in a long term extension study. Patients received meningococcal vaccination prior to receipt of Soliris. In all studies, the dose of Soliris was 600 mg study drug every 7 ± 2 days for 4 weeks, followed by 900 mg 7 ± 2 days later, then 900 mg every 14 ± 2 days for the study duration. Soliris was administered as an intravenous infusion over 25 - 45 minutes.
Study 1:
PNH patients with at least four transfusions in the prior 12 months, flow cytometric confirmation of at least 10% PNH cells and platelet counts of at least 100,000/microliter were randomized to either Soliris (n = 43) or placebo (n = 44). Prior to randomization, all patients underwent an initial observation period to confirm the need for RBC transfusion and to identify the hemoglobin concentration (the "set-point") which would define each patient's hemoglobin stabilization and transfusion outcomes. The hemoglobin set-point was less than or equal to 9 g/dL in patients with symptoms and was less than or equal to 7 g/dL in patients without symptoms. Endpoints related to hemolysis included the numbers of patients achieving hemoglobin stabilization, the number of RBC units transfused, fatigue, and health-related quality of life. To achieve a designation of hemoglobin stabilization, a patient had to maintain a hemoglobin concentration above the hemoglobin set-point and avoid any RBC transfusion for the entire 26 week period. Hemolysis was monitored mainly by the measurement of serum LDH levels, and the proportion of PNH RBCs was monitored by flow cytometry. Patients receiving anticoagulants and systemic corticosteroids at baseline continued these medications.
Major baseline characteristics were balanced (see Table 8).
| Parameter | Study 1 | |
|
Placebo N = 44 |
Soliris N = 43 |
|
| Mean age (SD) | 38 (13) | 42 (16) |
| Gender - female (%) | 29 (66) | 23 (54) |
| History of aplastic anemia or myelodysplastic syndrome (%) | 12 (27) | 8 (19) |
| Patients with history of thrombosis (events) | 8 (11) | 9 (16) |
| Concomitant anticoagulants (%) | 20 (46) | 24 (56) |
| Concomitant steroids/immunosuppressant treatments (%) | 16 (36) | 14 (33) |
| Packed RBC units transfused per patient in previous 12 months (median (Q1,Q3)) | 17 (14, 25) | 18 (12, 24) |
| Mean Hgb level (g/dL) at setpoint (SD) | 8 (1) | 8 (1) |
| Pre-treatment LDH levels (median, U/L) | 2,234 | 2,032 |
| Free hemoglobin at baseline (median, mg/dL) | 46 | 41 |
Patients treated with Soliris had significantly reduced (p< 0.001) hemolysis resulting in improvements in anemia as indicated by increased hemoglobin stabilization and reduced need for RBC transfusions compared to placebo treated patients (see Table 9). These effects were seen among patients within each of the three pre-study RBC transfusion strata (4 - 14 units; 15 - 25 units; > 25 units). After 3 weeks of Soliris treatment, patients reported less fatigue and improved health-related quality of life. Because of the study sample size and duration, the effects of Soliris on thrombotic events could not be determined.
Percentage of patients with stabilized hemoglobin levels
| Placebo | Soliris | |
| N = 44 | N = 43 | |
| Percentage of patients with stabilized hemoglobin levels | 0 | 49 |
| Packed RBC units transfused per patient (median) | 10 | 0 |
| (range) | (2 - 21) | (0 - 16) |
| Transfusion avoidance (%) | 0 | 51 |
| LDH levels at end of study (median, U/L) | 2,167 | 239 |
| Free hemoglobin at end of study (median, mg/dL) | 62 | 5 |
Study 2 and Extension Study:
PNH patients with at least one transfusion in the prior 24 months and at least 30,000 platelets/microliter received Soliris over a 52-week period. Concomitant medications included anti-thrombotic agents in 63% of the patients and systemic corticosteroids in 40% of the patients. Overall, 96 of the 97 enrolled patients completed the study (one patient died following a thrombotic event). A reduction in intravascular hemolysis as measured by serum LDH levels was sustained for the treatment period and resulted in a reduced need for RBC transfusion and less fatigue. 187 Soliris-treated PNH patients were enrolled in a long term extension study. All patients sustained a reduction in intravascular hemolysis over a total Soliris exposure time ranging from 10 to 54 months. There were fewer thrombotic events with Soliris treatment than during the same period of time prior to treatment. However, the majority of patients received concomitant anticoagulants; the effects of anticoagulant withdrawal during Soliris therapy was not studied [see Warnings and Precautions (5.4)].
14.2 aHUS
Five single-arm studies [four prospective (aHUS Studies 1, 2, 4 and 5) and one retrospective (aHUS Study 3)] evaluated the safety and efficacy of Soliris for the treatment of aHUS. Patients with aHUS received meningococcal vaccination prior to receipt of Soliris or received prophylactic treatment with antibiotics until 2 weeks after vaccination. In all studies, the dose of Soliris in adult and adolescent patients was 900 mg every 7 ± 2 days for 4 weeks, followed by 1200 mg 7 ± 2 days later, then 1200 mg every 14 ± 2 days thereafter. The dosage regimen for pediatric patients weighing less than 40 kg enrolled in aHUS Study 3 and Study 5 was based on body weight [see Dosage and Administration (2.2) ]. Efficacy evaluations were based on thrombotic microangiopathy (TMA) endpoints.
Endpoints related to TMA included the following:
- platelet count change from baseline
- hematologic normalization (maintenance of normal platelet counts and LDH levels for at least four weeks)
- complete TMA response (hematologic normalization plus at least a 25% reduction in serum creatinine for a minimum of four weeks)
- TMA-event free status (absence for at least 12 weeks of a decrease in platelet count of >25% from baseline, plasma exchange or plasma infusion, and new dialysis requirement)
- Daily TMA intervention rate (defined as the number of plasma exchange or plasma infusion interventions and the number of new dialyses required per patient per day).
aHUS Resistant to PE/PI (aHUS Study 1)
aHUS Study 1 enrolled patients who displayed signs of thrombotic microangiopathy (TMA) despite receiving at least four PE/PI treatments the week prior to screening. One patient had no PE/PI the week prior to screening because of PE/PI intolerance. In order to qualify for enrollment, patients were required to have a platelet count ≤150 x 109/L, evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 28 (range: 17 to 68 years). Patients enrolled in aHUS Study 1 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 70%-121%. Seventy-six percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 10 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 1.
| Parameter | aHUS Study 1
N = 17 |
| Time from aHUS diagnosis until screening in months, median (min, max) | 10 (0.26, 236) |
| Time from current clinical TMA manifestation until screening in months, median (min, max) | <1 (<1, 4) |
| Baseline platelet count (× 109/L), median (range) | 118 (62, 161) |
| Baseline LDH (U/L), median (range) | 269 (134, 634) |
Patients in aHUS Study 1 received Soliris for a minimum of 26 weeks. In aHUS Study 1, the median duration of Soliris therapy was approximately 100 weeks (range: 2 weeks to 145 weeks).
Renal function, as measured by eGFR, was improved and maintained during Soliris therapy. The mean eGFR (± SD) increased from 23 ± 15 mL/min/1.73m2 at baseline to 56 ± 40 mL/min/1.73m2 by 26 weeks; this effect was maintained through 2 years (56 ± 30 mL/min/1.73m2). Four of the five patients who required dialysis at baseline were able to discontinue dialysis.
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of Soliris. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 1, mean platelet count (± SD) increased from 109 ± 32 x109/L at baseline to 169 ± 72 x109/L by one week; this effect was maintained through 26 weeks (210 ± 68 x109/L), and 2 years (205 ± 46 x109/L). When treatment was continued for more than 26 weeks, two additional patients achieved Hematologic Normalization as well as Complete TMA response. Hematologic Normalization and Complete TMA response were maintained by all responders. In aHUS Study 1, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins.
Table 11 summarizes the efficacy results for aHUS Study 1.
| Efficacy Parameter |
aHUS Study 1 at 26 wks1
N = 17 |
aHUS Study 1 at 2 yrs2 N = 17 |
| Complete TMA response, n (%) Median Duration of complete TMA response, weeks (range) |
11 (65) 38 (25, 56) |
13 (77) 99 (25, 139) |
| eGFR improvement ≥15 mL/min/1.73 m2, n (%) Median duration of eGFR improvement, days (range) |
9 (53) 251 (70, 392) |
10 (59) ND |
| Hematologic normalization, n (%) Median Duration of hematologic normalization, weeks (range) |
13 (76) 37 (25, 62) |
15 (88) 99 (25, 145) |
| TMA event-free status, n (%) | 15 (88) | 15 (88) |
| Daily TMA intervention rate, median (range) Before eculizumab On eculizumab treatment |
0.82 (0.04, 1.52) 0 (0, 0.31) |
0.82 (0.04, 1.52) 0 (0, 0.36) |
1.At data cut-off (September 8, 2010).
2.At data cut-off (April 20, 2012).
aHUS Sensitive to PE/PI (aHUS Study 2)
aHUS Study 2 enrolled patients undergoing chronic PE/PI who generally did not display hematologic signs of ongoing thrombotic microangiopathy (TMA). All patients had received PT at least once every two weeks, but no more than three times per week, for a minimum of eight weeks prior to the first Soliris dose. Patients on chronic dialysis were permitted to enroll in aHUS Study 2. The median patient age was 28 years (range: 13 to 63 years). Patients enrolled in aHUS Study 2 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 37%-118%. Seventy percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 12 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 2.
| Parameter | aHUS Study 2
N=20 |
| Time from aHUS diagnosis until screening in months, median (min, max) | 48 (0.66, 286) |
| Time from current clinical TMA manifestation until screening in months, median (min, max) | 9 (1, 45) |
| Baseline platelet count (× 109/L), median (range) | 218 (105, 421) |
| Baseline LDH (U/L), median (range) | 200 (151, 391) |
Patients in aHUS Study 2 received Soliris for a minimum of 26 weeks. In aHUS Study 2, the median duration of Soliris therapy was approximately 114 weeks (range: 26 to 129 weeks).
Renal function, as measured by eGFR, was maintained during Soliris therapy. The mean eGFR (± SD) was 31 ± 19 mL/min/1.73m2 at baseline, and was maintained through 26 weeks (37 ± 21 mL/min/1.73m2) and 2 years (40 ± 18 mL/min/1.73m2). No patient required new dialysis with Soliris.
Reduction in terminal complement activity was observed in all patients after the commencement of Soliris. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. Platelet counts were maintained at normal levels despite the elimination of PE/PI. The mean platelet count (± SD) was 228 ± 78 x 109/L at baseline, 233 ± 69 x 109/L at week 26, and 224 ± 52 x 109/L at 2 years. When treatment was continued for more than 26 weeks, six additional patients achieved Complete TMA response. Complete TMA Response and Hematologic Normalization were maintained by all responders. In aHUS Study 2, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins.
Table 13 summarizes the efficacy results for aHUS Study 2.
|
Efficacy Parameter |
aHUS Study 2 at 26 wks1 N = 20 |
aHUS Study 2 at 2 yrs2 N = 20 |
|
Complete TMA response, n (%) Median duration of complete TMA response, weeks (range) |
5 (25) 32 (12, 38) |
11 (55) 68 (38, 109) |
|
eGFR improvement ≥15 mL/min/1.73 m2, n (%) |
1 (5) |
8 (40) |
|
TMA Event-free status n (%) |
16 (80) |
19 (95) |
|
Daily TMA intervention rate, median (range) Before eculizumab On eculizumab treatment |
0 |
0.23 (0.05, 1.07) 0 (0, 0.01) |
|
Hematologic normalization4, n (%) Median duration of hematologic normalization, weeks (range)3 |
18 (90) 38 (22, 52) |
18 (90) 114 (33, 125) |
1. At data cut-off (September 8, 2010).
2. At data cut-off (April 20, 2012).
3. Calculated at each post-dose day of measurement (excluding Days 1 to 4) using a repeated measurement ANOVA model.
4. In aHUS Study 2, 85% of patients had normal platelet counts and 80% of patients had normal serum LDH levels at baseline, so hematologic normalization in this population reflects maintenance of normal parameters in the absence of PE/PI.
Retrospective Study in Patients with aHUS (aHUS Study 3)
The efficacy results for the aHUS retrospective study (aHUS Study 3) were generally consistent with results of the two prospective studies. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline. Mean platelet count (± SD) increased from 171 ± 83 x109/L at baseline to 233 ±109 x109/L after one week of therapy; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 254 ± 79 x109/L).
A total of 19 pediatric patients (ages 2 months to 17 years) received Soliris in aHUS Study 3. The median duration of Soliris therapy was 16 weeks (range 4 to 70 weeks) for children <2 years of age (n=5), 31 weeks (range 19 to 63 weeks) for children 2 to <12 years of age (n=10), and 38 weeks (range 1 to 69 weeks) for patients 12 to <18 years of age (n=4). Fifty three percent of pediatric patients had an identified complement regulatory factor mutation or auto-antibody.
Overall, the efficacy results for these pediatric patients appeared consistent with what was observed in patients enrolled in aHUS Studies 1 and 2 (Table 14). No pediatric patient required new dialysis during treatment with Soliris.
|
Efficacy Parameter |
<2 yrs (n=5) |
2 to <12 yrs (n=10) |
12 to <18 yrs (n=4) |
Total (n=19) |
|
Complete TMA response, n (%) |
2 (40) |
5 (50) |
1 (25) |
8 (42) |
|
Patients with eGFR improvement ≥ 15 mL/min/1.73m2, n (%)2 |
2 (40) |
6 (60) |
1 (25) |
9 (47) |
|
Platelet count normalization, n (%)1 |
4 (80) |
10 (100) |
3 (75) |
17 (89) |
|
Hematologic Normalization, n (%) |
2 (40) |
5 (50) |
1 (25) |
8 (42) |
|
Daily TMA intervention rate, median (range) Before eculizumab On eculizumab treatment |
1 (0, 2) <1 (0, <1) |
<1 (0.07, 1.46) 0 (0, <1) |
<1 (0, 1) 0 (0, <1) |
0.31 (0.00, 2.38) 0.00 (0.00 , 0.08) |
1. Platelet count normalization was defined as a platelet count of at least 150,000 X 109/L on at least two consecutive measurements spanning a period of at least 4 weeks.
2. Of the 9 patients who experienced an eGFR improvement of at least 15 mL/min/1.73 m2, one received dialysis throughout the study period and another received Soliris as prophylaxis following renal allograft transplantation.
Adult Patients with aHUS (aHUS Study 4)
aHUS Study 4 enrolled patients who displayed signs of thrombotic microangiopathy (TMA). In order to qualify for enrollment, patients were required to have a platelet count < lower limit of normal range (LLN), evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 35 (range: 18 to 80 years). All patients enrolled in aHUS Study 4 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 28%-116%. Fifty-one percent of patients had an identified complement regulatory factor mutation or auto-antibody. A total of 35 patients received PE/PI prior to eculizumab. Table 15 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 4.
|
Parameter |
aHUS Study 4 N = 41 |
|
Time from aHUS diagnosis until start of study drug in months, median (range) |
0.79 (0.03 – 311) |
|
Time from current clinical TMA manifestation until first study dose in months, median (range) |
0.52 (0.03-19) |
|
Baseline platelet count (× 109/L), median (range) |
125 (16 – 332) |
|
Baseline LDH (U/L), median (range) |
375 (131 – 3318) |
Patients in aHUS Study 4 received Soliris for a minimum of 26 weeks. In aHUS Study 4, the median duration of Soliris therapy was approximately 50 weeks (range: 13 weeks to 86 weeks).
Renal function, as measured by eGFR, was improved during Soliris therapy. The mean eGFR (± SD) increased from 17 ± 12 mL/min/1.73m2 at baseline to 47 ± 24 mL/min/1.73m2 by 26 weeks. Twenty of the 24 patients who required dialysis at study baseline were able to discontinue dialysis during Soliris treatment.
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of Soliris. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 4, mean platelet count (± SD) increased from 119 ± 66 x109/L at baseline to 200 ± 84 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 252 ± 70 x109/L). In aHUS Study 4, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H.
Table 16 summarizes the efficacy results for aHUS Study 4.
|
Efficacy Parameter |
aHUS Study 4 (N = 41) |
|
Complete TMA response, n (%), 95% CI Median duration of complete TMA response, weeks (range) |
23 (56), 40,72 42 (6, 75) |
|
Patients with eGFR improvement ≥ 15 mL/min/1.73 m2, n (%) |
22 (54) |
|
Hematologic Normalization, n (%) Median duration of hematologic normalization, weeks (range) |
36 (88) 46 (10, 75) |
|
TMA Event-free Status, n (%) |
37 (90) |
|
Daily TMA Intervention Rate, median (range) Before eculizumab On eculizumab treatment |
0.63 (0, 1.38) 0 (0, 0.58) |
Pediatric and Adolescent Patients with aHUS (aHUS Study 5)
aHUS Study 5 enrolled patients who were required to have a platelet count < lower limit of normal range (LLN), evidence of hemolysis such as an elevation in serum LDH above the upper limits of normal, serum creatinine level ≥97 percentile for age without the need for chronic dialysis. The median patient age was 6.5 (range: 5 months to 17 years). Patients enrolled in aHUS Study 5 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 38%-121%. Fifty percent of patients had an identified complement regulatory factor mutation or auto-antibody. A total of 10 patients received PE/PI prior to eculizumab. Table 17 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 5.
|
Parameter |
Patients 1 month to <12 years (N = 18) |
All Patients (N = 22) |
|
Time from aHUS diagnosis until start of study drug in months, median (range) |
0.51 (0.03 – 58) |
0.56 (0.03-191) |
|
Time from current clinical TMA manifestation until first study dose in months, median (range) |
0.23 (0.03 – 4) |
0.2 (0.03-4) |
|
Baseline platelet count (x 109/L), median (range) |
110 (19-146) |
91 (19-146) |
|
Baseline LDH (U/L) median (range) |
1510 (282-7164) |
1244 (282-7164) |
Patients in aHUS Study 5 received Soliris for a minimum of 26 weeks. In aHUS Study 5, the median duration of Soliris therapy was approximately 44 weeks (range: 1 dose to 88 weeks).
Renal function, as measured by eGFR, was improved during Soliris therapy. The mean eGFR (± SD) increased from 33 ± 30 mL/min/1.73m2 at baseline to 98 ± 44 mL/min/1.73m2 by 26 weeks. Among the 20 patients with a CKD stage ≥2 at baseline, 17 (85%) achieved a CKD improvement of ≥1 stage. Among the 16 patients ages 1 month to <12 years with a CKD stage ≥2 at baseline, 14 (88%) achieved a CKD improvement by ≥1 stage. Nine of the 11 patients who required dialysis at study baseline were able to discontinue dialysis during Soliris treatment. Responses were observed across all ages from 5 months to 17 years of age.
Reduction in terminal complement activity was observed in all patients after commencement of Soliris. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. The mean platelet count (± SD) increased from 88 ± 42 x109/L at baseline to 281 ± 123 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (±SD) at week 26: 293 ± 106 x109/L). In aHUS Study 5, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H.
Table 18 summarizes the efficacy results for aHUS Study 5.
|
Efficacy Parameter |
Patients
1 month to <12 (N = 18) |
All Patients (N = 22) |
|
Complete TMA response, n (%) 95% CI Median Duration of complete TMA response, weeks (range)1 |
11 (61) 36, 83 40 (14, 77) |
14 (64) 41, 83 37 (14, 77) |
|
eGFR improvement ≥15 mL/min/ 1.73•m2•n (%) |
16 (89) |
19 (86) |
|
Complete Hematologic Normalization, n (%) Median Duration of complete hematologic normalization, weeks (range) |
14 (78) 38 (14, 77) |
18 (82) 38 (14, 77) |
|
TMA Event-Free Status, n (%) |
17 (94) |
21 (95) |
|
Daily TMA Intervention rate, median (range) Before eculizumab treatment On eculizumab treatment |
0.2 (0, 1.7) 0 (0, 0.01) |
0.4 (0, 1.7) 0 (0, 0.01) |
1. through data cutoff (October 12, 2012).
16 HOW SUPPLIED/STORAGE AND HANDLING
Soliris (eculizumab) is supplied as 300 mg single-use vials containing 30 mL of 10 mg/mL sterile, preservative-free Soliris solution per vial.
Soliris vials must be stored in the original carton until time of use under refrigerated conditions at 2-8º C (36-46º F) and protected from light. Do not use beyond the expiration date stamped on the carton. Refer to [ Dosage and Administration (2) ] for information on the stability and storage of diluted solutions of Soliris.
DO NOT FREEZE. DO NOT SHAKE.
NDC 25682-001-01 Single unit 300 mg carton: Contains one (1) 30 mL vial of Soliris (10 mg/mL).
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
Prior to treatment, patients should fully understand the risks and benefits of Soliris, in particular the risk of meningococcal infection. Ensure that patients receive the Medication Guide.
Inform patients that they are required to receive a meningococcal vaccination at least 2 weeks prior to receiving the first dose of Soliris, if they have not previously been vaccinated. They are required to be revaccinated according to current medical guidelines for meningococcal vaccine use while on Soliris therapy. Inform patients that vaccination may not prevent meningococcal infection. Inform patients about the signs and symptoms of meningococcal infection, and strongly advise patients to seek immediate medical attention if these signs or symptoms occur. These signs and symptoms are as follows:
- headache with nausea or vomiting
- headache and a fever
- headache with a stiff neck or stiff back
- fever of 103° F (39.4° C) or higher
- fever and a rash
- confusion
- muscle aches with flu-like symptoms
- eyes sensitive to light
Inform patients that they will be given a Soliris Patient Safety Information Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation.
Inform patients that there may be an increased risk of other types of infections, particularly those due to encapsulated bacteria. Additionally, Aspergillus infections have occured in immunocompromised and neutropenic patients. Inform parents or caregivers of children receiving Soliris for the treatment of aHUS that their child should be vaccinated against Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to current medical guidelines.
Inform patients with PNH that they may develop hemolysis due to PNH when Soliris is discontinued and that they will be monitored by their healthcare professional for at least 8 weeks following Soliris discontinuation. Inform patients with aHUS that there is a potential for TMA complications due to aHUS when Soliris is discontinued and that they will be monitored by their healthcare professional for at least 12 weeks following Soliris discontinuation. Inform patients who discontinue Soliris to keep the Soliris Patient Safety Information Card with them for three months after the last Soliris dose, because the increased risk of meningococcal infection persists for several weeks following discontinuation of Soliris.
Manufactured by:
Alexion Pharmaceuticals, Inc.
352 Knotter Drive
Cheshire, CT 06410 USA
US License Number 1743
This product, or its use, may be covered by one or more US patents, including US Patent No. 6,355,245 in addition to others including patents pending.
MEDICATION GUIDE
Soliris® (so-leer-is)
(eculizumab)
Read the Medication Guide before you start Soliris and before each infusion. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment. Talk to your doctor if you have any questions about your treatment with Soliris.
What is the most important information I should know about Soliris?
Soliris is a medicine that affects your immune system. Soliris can lower the ability of your immune system to fight infections.
- Soliris increases your chance of getting serious and life-threatening meningococcal infections.
Meningococcal infections may quickly become life-threatening and cause death if not recognized and treated early.
- You must receive a meningococcal vaccine at least 2 weeks before your first dose of Soliris unless you have already had this vaccine. If your doctor decided that urgent treatment with Soliris is needed, you should receive a meningococcal vaccine as soon as possible.
- If you had a meningococcal vaccine in the past, you might need a booster dose before starting Soliris. Your doctor will decide if you need another dose of a meningococcal vaccine.
-
A meningococcal vaccine does not prevent all meningococcal infections. Call your doctor or get emergency medical care right away if you get any of these signs and symptoms of a meningococcal infection:
- headache with nausea or vomiting
- headache and a fever
- headache with a stiff neck or stiff back
- fever of 103° F (39.4° C) or higher
- fever and a rash
- confusion
- muscle aches with flu-like symptoms
- eyes sensitive to light
Your doctor will give you a Patient Safety Card about the risk of meningococcal infection. Carry it with you at all times during treatment and for 3 months after your last Soliris dose. Your risk of meningococcal infection may continue for several weeks after your last dose of Soliris. It is important to show this card to any doctor or nurse who treats you. This will help them diagnose and treat you quickly.
Soliris is only available through a program called the Soliris REMS. Before you can receive Soliris, your doctor must:
- enroll in the Soliris REMS program
- counsel you about the risk of meningococcal infection
- give you information about the symptoms of meningococcal infection
- give you a Patient Safety Card about your risk of meningococcal infection, as discussed above
- make sure that you are vaccinated with a meningococcal vaccine
Soliris may also increase the risk of other types of serious infections. If your child is treated with Soliris, make sure that your child receives vaccinations against Streptococcus pneumoniae and Haemophilis influenza type b (Hib).
What Is Soliris?
Soliris is a prescription medicine called a monoclonal antibody. Soliris is used to treat people with:
- a disease called Paroxysmal Nocturnal Hemoglobinuria (PNH). PNH affects red blood cells.
- A disease called atypical Hemolytic Uremic Syndrome (aHUS). aHUS affects the blood system, kidney, and sometimes other body organs.
Soliris works by blocking part of your immune system. This can help your symptoms but it can also increase your chance for infection.
It is important that you:
- have all recommended vaccinations before you start Soliris
- stay up-to-date with all recommended vaccinations during treatment with Soliris
Who Should Not Receive Soliris?
Do not receive Soliris if you:
- have a meningococcal infection
- have not been vaccinated against meningitis infection unless your doctor decides that urgent treatment with Soliris is needed. See “What is the most important information I should know about Soliris?”
What should I tell my doctor before receiving Soliris?
Before receiving Soliris, tell your doctor if you:
- have an infection or fever
- are pregnant or plan to become pregnant. It is not known if Soliris will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Soliris passes into your breast milk.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medications you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How will I receive Soliris?
- Soliris is given through a vein (I.V. or intravenous infusion) usually over 35 minutes in adults and 1-4 hours in pediatric patients. If you have an allergic reaction during your Soliris infusion, your doctor may decide to give Soliris more slowly or stop your infusion.
- If you are an adult, you will usually receive a Soliris infusion by your doctor:
- weekly for five weeks, the
- every 2 weeks
- If you are less than 18 years of age, your doctor will decide how often you will receive Soliris depending on your age and body weight
- After each infusion, you should be monitored for one hour for allergic reactions. See “What are the possible side effects of Soliris?”
- If you forget or miss a Soliris infusion, call your doctor right away.
- If you have PNH, your doctor will need to monitor you closely for at least 8 weeks after stopping Soliris. Stopping treatment with Soliris may cause breakdown of your red blood cells due to PNH.
Symptoms or problems that can happen due to red blood cell breakdown include:
o drop in the number of your red blood cell count
o drop in your platelet count
o confusion
o chest pain
o kidney problems
o blood clots
o difficulty breathing
- If you have aHUS, your doctor will need to monitor you closely during and for at least 12 weeks after stopping treatment for signs of worsening aHUS symptoms or problems related to abnormal clotting (thrombotic microangiopathy).
Symptoms or problems that can happen with abnormal clotting may include:
o stroke
o confusion
o seizures
o chest pain (angina)
o difficulty breathing
o kidney problems
o swelling in arms or legs
o a drop in your platelet count
What are the possible side effects of Soliris?
Soliris can cause serious side effects including:
- See “What is the most important information I should know about Soliris?”
- Serious allergic reactions. Serious allergic reactions can happen during your Soliris infusion. Tell your doctor or nurse right away if you get any of these symptoms during your Soliris infusion:
- chest pain
- trouble breathing or shortness of breath
- swelling of your face, tongue, or throat
- feel faint or pass out
If you have an allergic reaction to Soliris, your doctor may need to infuse Soliris more slowly, or stop Soliris. See “How will I receive Soliris?”
Common side effects in people with PNH treated with Soliris include:
- headaches
- runny nose and colds
- sore throat
- back pain
- nausea
Common side effects in people with aHUS treated with Soliris include:
- headache
- diarrhea
- hypertension
- common cold (upper respiratory infection)
- abdominal pain
- vomiting
- nasopharyngitis
- anemia
- cough
- peripheral edema
- nausea
- urinary tract infections
- pyrexia
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of Soliris. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about Soliris
Medicines are sometimes prescribed for conditions other than those listed in a Medication Guide. This Medication Guide summarizes the most important information about Soliris. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Soliris that is written for healthcare professionals.
What are the ingredients in Soliris?
Active ingredient: eculizumab
Inactive ingredients: sodium phosphate monobasic, sodium phosphate dibasic, sodium chloride, polysorbate 80 (vegetable origin) and Water for Injection
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by Alexion Pharmaceuticals, Inc., 352 Knotter Drive, Cheshire, CT 06410 USA.
Revised: 04/2014
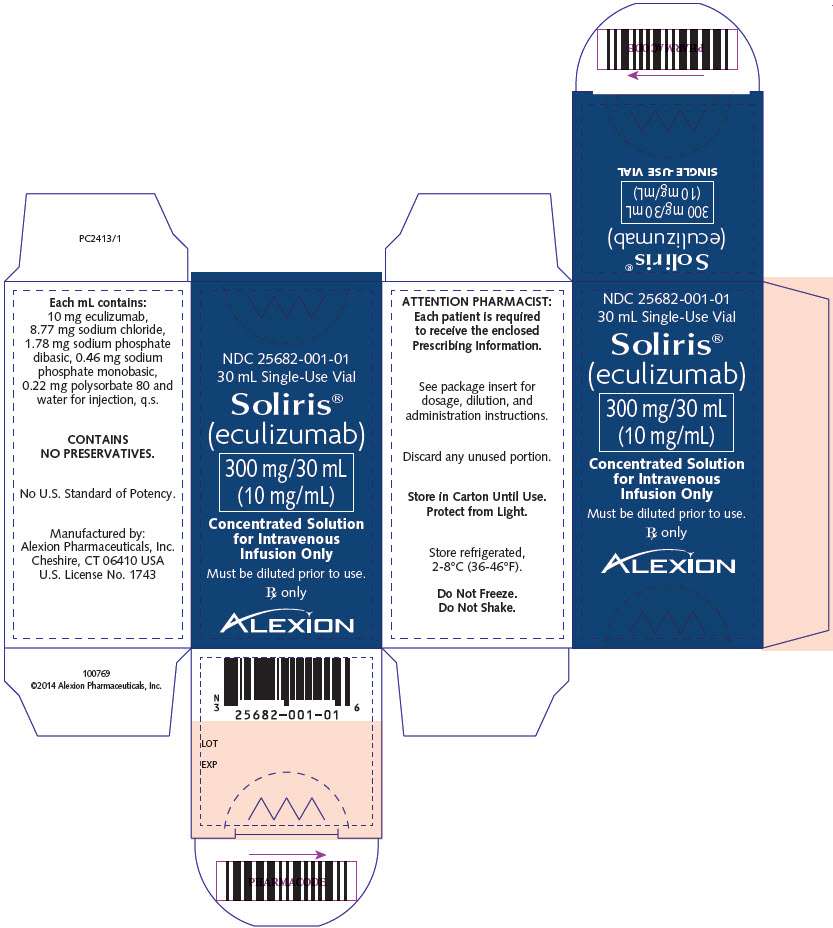
PRINCIPAL DISPLAY PANEL
NDC 25682-001-01
30 mL Single-Use Vial
Soliris®
(eculizumab)
300 mg/30 mL
(10 mg/mL)
Concentrated Solution
for Intravenous
Infusion Only
Must be diluted prior to use.
Rx only
Soliriseculizumab INJECTION, SOLUTION, CONCENTRATE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||