Solbar Zinc SPF38
Solbar Zinc
FULL PRESCRIBING INFORMATION: CONTENTS*
- Indications and use
- Purpose
- Keep out of the reach of children
- Dosage and Administration
- Warnings
- OTC - ACTIVE INGREDIENT SECTION
- INACTIVE INGREDIENT SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Indications and use
Helps prevent sunburn.
Purpose
Sunscreen
Keep out of the reach of children
Yes. If swallowed, get medical help or contact a Poison Control Center right away.
Dosage and Administration
Apply liberally and evenly to all sun exposed areas of DRY skin 15 minutes before sun exposure. Reapply after 80 minutes of swimming or sweating and immediately after towel drying. Apply at least every 2 hours. For children under 6 months, ask a physician.
Warnings
For external use only. Do not use on damaged or broken skin. Keep out of eyes. Rinse eyes thoroughly with water to remove. Stop use and ask a physician if rash or irritation develops and lasts. Store away from excessive heat and direct sun.
OTC - ACTIVE INGREDIENT SECTION
Homosalate
Octinoxate
Zinc Oxide
INACTIVE INGREDIENT SECTION
Water
Isobutyl Stearate
PEG-100 Stearate
Glycerin
Dimethicone
PVP/Eicosene Copolymer
Glyceryl Dilaurate
Cetyl Alcohol
DEA Cetyl Phosphate
Benzyl Alcohol
Cyclomethicone
Stearyl Alcohol
Xanthan Gum
Disodium EDTA
Citric Acid
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Solbar Zinc.jpg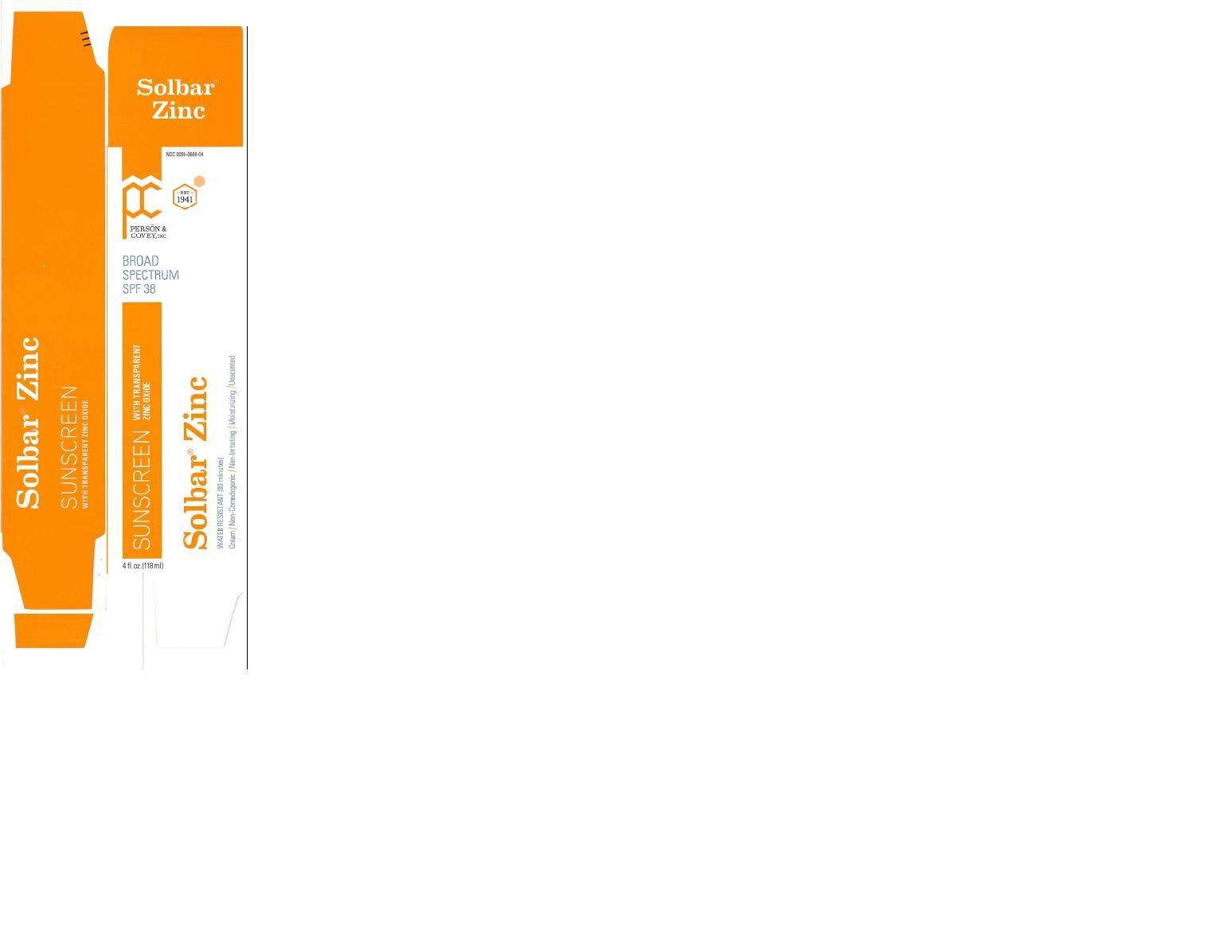
Solbar Zinc SPF38Solbar Zinc SPF38 CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||