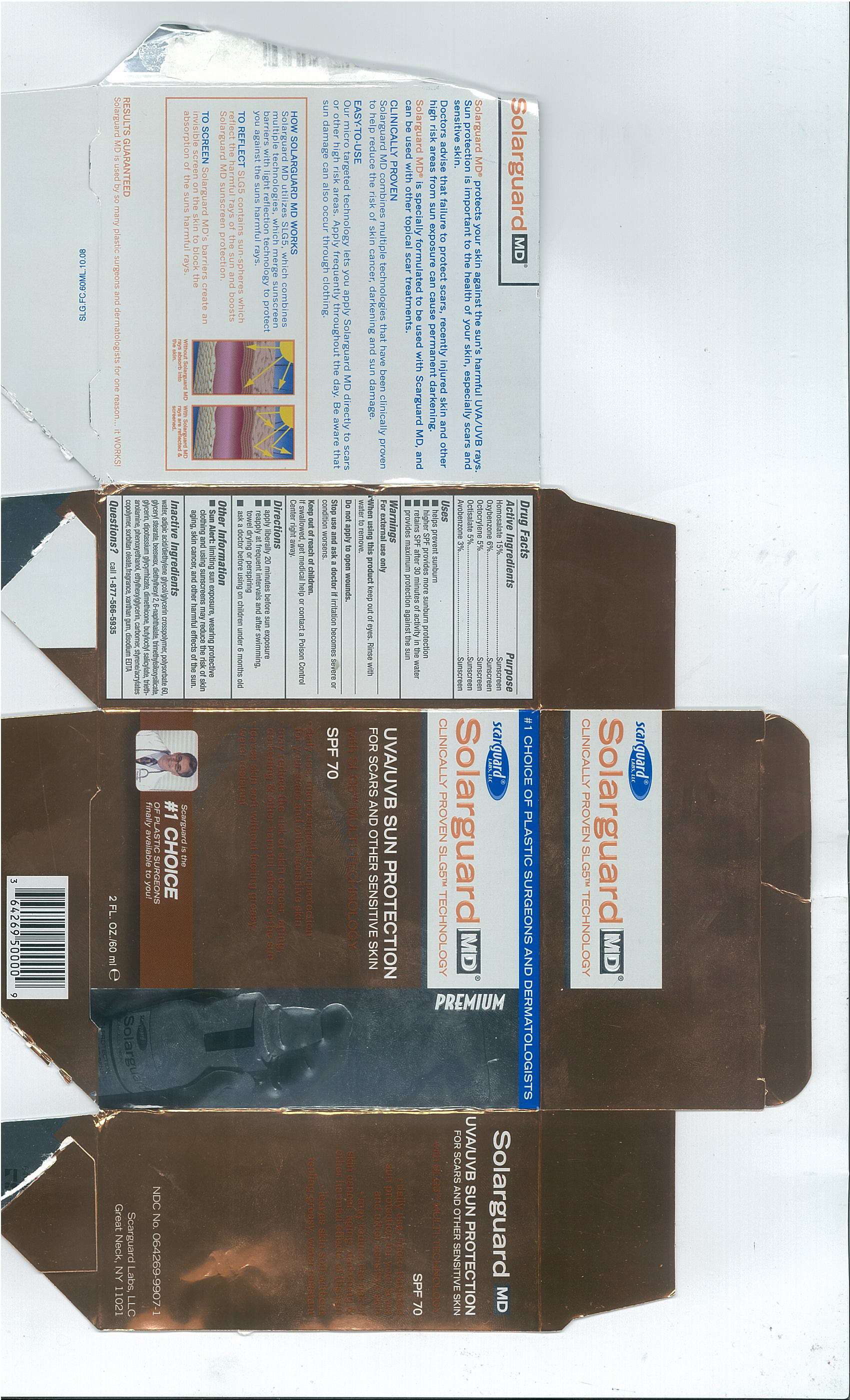
Solarguard MD
Scarguard Labs, LLC
Scarguard Labs, LLC
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Solarguard MD Uses
- Warnings
- Keep out of reach of children
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- 60 mL package
FULL PRESCRIBING INFORMATION
Active Ingredients
Homosalate 15%
Oxybenzone 6%
Octocrylene 5%
Octisalate 5%
Avobenzone 3%
Purpose
Solarguard MD Uses
- helps prevent sunburn
- high SPF provides more sunburn protection
- retains SPF after 30 minutes of activity in the water
- provides maximum protection against the sun
Warnings
For external use only
When using this product keep out of eyes. Rinse with water to remove.
Do not apply to open wounds.
Stop use and ask a doctor if irritation becomes severe or condition worsens.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control
Center right away.
Directions
- apply liberally 20 minutes before sun exposure
- reapply at frequent intervals and after swimming, towel drying or perspiring
- ask a doctor before using on children under 6 months old
Other Information
-
Sun Alert: limiting sun exposure, wearing protective clothing and using sunscreens may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive Ingredients
water, adipic acid/diethylene glycol/glycerin crosspolymer, polysorbate 60, glyceryl stearate, beeswax, diethylhexyl 2,6-naphthalate, trimethylsiloxysilicate,glycerin, dipotassium glycyrrhizate, dimethicone, butyoctyl salicylate, triethanolamine, phenoxyethanol ethylhexylgylcerin, carbomer, styrene/acrylates copolymer, sorbitan oleate, fragrance, xanthan gum, disodium EDTA
Questions?
call 1-877-566-5935
60 mL package
Solarguard MDHomosalate Oxybenzone Octocrylene Octisalate Avobenzone SPRAY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||