Sodium Chloride
0.9% Sodium Chloride Injection, USPin Mini-Bag™ Plus Container Viaflex® Plastic Container
FULL PRESCRIBING INFORMATION: CONTENTS*
- SODIUM CHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- SODIUM CHLORIDE INDICATIONS AND USAGE
- SODIUM CHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SODIUM CHLORIDE ADVERSE REACTIONS
- SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPLE DISPLAY PANEL
FULL PRESCRIBING INFORMATION
SODIUM CHLORIDE DESCRIPTION
0.9% Sodium Chloride Injection, USP in the Mini-Bag™ Plus Container is a sterile, nonpyrogenic solution for intravenous administration after admixture with a single dose powdered drug. It contains no antimicrobial agents. Each 100 mL contains 900 mg of Sodium Chloride, USP (NaCl). The osmolarity is 308 mOsmol/L (calculated). The pH is 5.0 (4.5 to 7.0). It contains 154 mEq/L of sodium and 154 mEq/L of chloride.
The Mini-Bag™ Plus Container is a standard diluent container with an integral drug vial adaptor. It allows for drug admixture after connection to a single dose powdered drug vial having a 20 mm closure. A breakaway seal in the tube between the vial adaptor and the container is broken to allow transfer of the diluent into the vial and reconstitution of the drug. The reconstituted drug is then transferred from the vial into the container diluent and mixed to result in an admixture for delivery to the patient.
The Viaflex® plastic container is fabricated from polyvinyl chloride (PL 146® Plastic). Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
CLINICAL PHARMACOLOGY
Sodium Chloride Injection, USP has value as a source of water and electrolytes. It is capable of inducing diuresis depending on the clinical condition of the patient.
SODIUM CHLORIDE INDICATIONS AND USAGE
0.9% Sodium Chloride Injection, USP is indicated as a source of water and electrolytes and may also be used as diluent for reconstitution of a powdered drug product packaged in a vial with a 20 mm closure.
SODIUM CHLORIDE CONTRAINDICATIONS
None known.
WARNINGS
Sodium Chloride Injection, USP should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of Sodium Chloride Injection, USP may result in sodium retention.
For use only with a single dose powdered drug vial with a 20 mm closure.
Do not administer unless drug is completely dissolved and drug vial is empty.
Additives may be incompatible.
Do not remove drug vial at any time prior to or during administration.
PRECAUTIONS
General
Do not administer unless solution is clear and all seals are intact
Laboratory Tests
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Drug Interactions
Caution must be exercised in the administration of Sodium Chloride Injection, USP to patients receiving corticosteroids or corticotropin.
Carcinogenesis and Mutagenesis and Impairment of Fertility
Studies with 0.9% Sodium Chloride Injection, USP have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Sodium Chloride Injection, USP. It is also not known whether Sodium Chloride Injection, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Chloride Injection, USP should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Caution should be exercised when 0.9% Sodium Chloride Injection, USP is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates and very small infants, the volume of fluid may affect fluid and electrolyte balance.
SODIUM CHLORIDE ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation, and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
As directed by a physician. Dosage is dependent upon the age, weight and clinical condition of the patient as well as laboratory determinations.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
All injections in Mini-Bag™ Plus containers are intended for intravenous administration using sterile equipment.
Do not remove unit from overwrap until ready for use. The overwrap is a moisture barrier.
To Open
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity should diminish gradually.
Prior to use, check that the vial adaptor cover is intact. Check the solution container for minute leaks by squeezing inner bag firmly. If leaks are found or if the vial adaptor cover is not intact, discard product as sterility may be impaired.
To Assemble and Reconstitute
See other side for detailed instructions.
Additives may be incompatible.
HOW SUPPLIED
0.9% Sodium Chloride Injection, USP in Mini-Bag™ Plus Container is available as follows:
| Code | Size (mL) | NDC |
| 2B0042 | 50 | 0338-0553-11 |
| 2B0043 | 100 | 0338-0553-18 |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C/77°F).
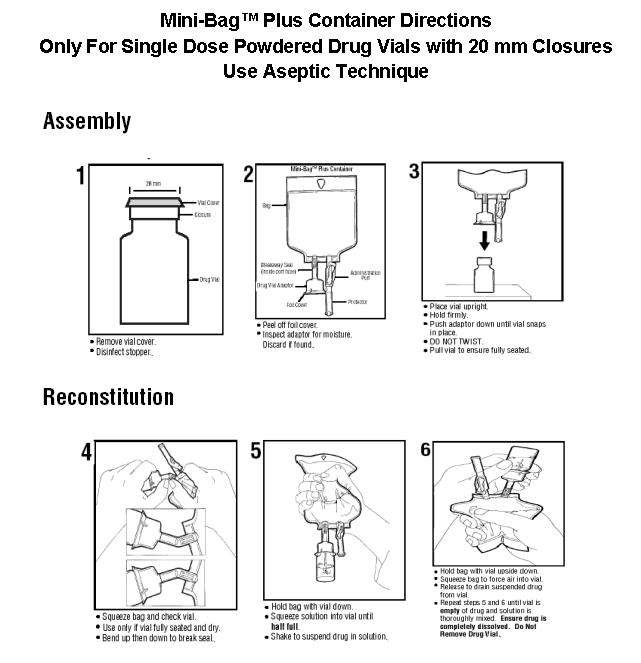
7. Remove port protector. Attach administration set per its directions.
8. Hang container on I.V. pole and prime set per directions. Ensure that vial is empty of drug and solution. Repeat step 6 if drug and solution remain in vial.
Warning: Do not use in series connections.
9. Administer medication per directions. Use within specified time for drug stability. Refer to drug package insert.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
©Copyright 1990, 1991, 1992, 1995, Baxter Healthcare Corporation. All rights reserved.
07-19-04-589
Rev. June 1999
PACKAGE LABEL.PRINCIPLE DISPLAY PANEL

Lot:PXX Exp: XX XX
QTY: 80-50 mL 2B0042
NDC: 0338-0553-11
0.9% SODIUM CHLORIDE INJECTION, USP
IN MINI-BAG PLUS CONTAINER
(17) XXXX00 (10) PXX
(01) 50303380553117
Sodium ChlorideSodium Chloride INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||