SHISEIDO WHITE LUCENT
SHISEIDO WHITE LUCENT BRIGHTENING PROTECTIVE EMULSION wSPF 15•PA++
FULL PRESCRIBING INFORMATION: CONTENTS*
- SHISEIDO WHITE LUCENT Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
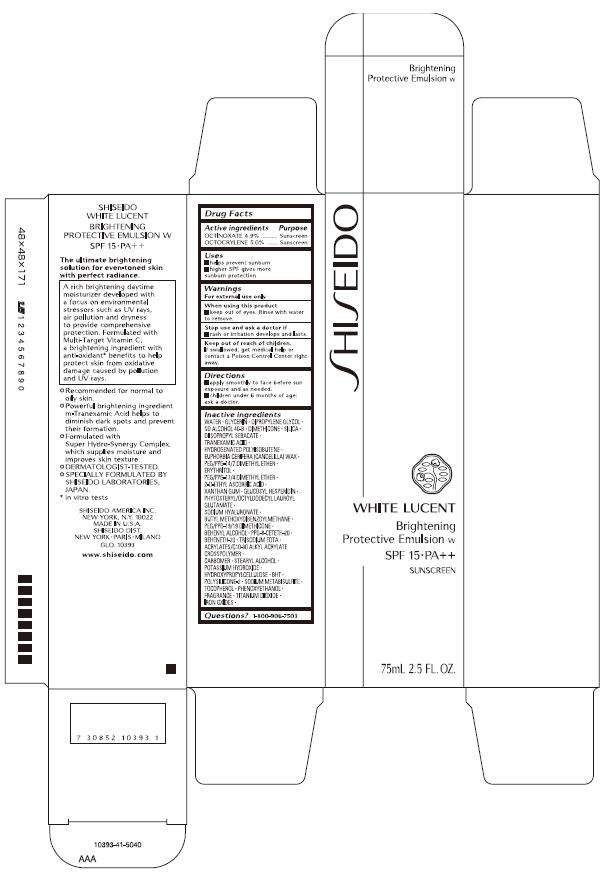
- PRINCIPAL DISPLAY PANEL - 75 mL Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
| OCTINOXATE 4.9% | Sunscreen |
| OCTOCRYLENE 5.0% | Sunscreen |
SHISEIDO WHITE LUCENT Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply smoothly to face before sun exposure and as needed.
- children under 6 months of age: ask a doctor.
Inactive ingredients
WATER, GLYCERIN, DIPROPYLENE GLYCOL, SD ALCOHOL 40-B, DIMETHICONE, SILICA, DIISOPROPYL SEBACATE, TRANEXAMIC ACID, HYDROGENATED POLYISOBUTENE, EUPHORBIA CERIFERA (CANDELILLA) WAX, PEG/PPG-14/7 DIMETHYL ETHER, ERYTHRITOL, PEG/PPG-17/4 DIMETHYL ETHER, 2-O-ETHYL ASCORBIC ACID, XANTHAN GUM GLUCOSYL HESPERIDIN, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, SODIUM HYALURONATE, BUTYL METHOXYDIBENZOYLMETHANE PEG/PPG-19/19 DIMETHICONE, BEHENYL ALCOHOL, PPG-8-CETETH-20, BEHENETH-20, TRISODIUM EDTA, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, CARBOMER, STEARYL ALCOHOL, POTASSIUM HYDROXIDE, HYDROXYPROPYLCELLULOSE, BHT, POLYSILICONE-2, SODIUM METABISULFITE, TOCOPHEROL, PHENOXYETHANOL, FRAGRANCE, TITANIUM DIOXIDE, IRON OXIDES
Questions?
1-800-906-7503
SHISEIDO AMERICA INC.
NEW YORK, N.Y. 10022
MADE IN U.S.A.
SHISEIDO DIST.
NEW YORK • PARIS • MILANO
GLO. 10393
www.shiseido.com
PRINCIPAL DISPLAY PANEL - 75 mL Carton
SHISEIDO
WHITE LUCENT
Brightening
Protective Emulsion w
SPF 15 • PA++
SUNSCREEN
75mL 2.5 FL. OZ.
SHISEIDO WHITE LUCENTOctinoxate and Octocrylene EMULSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||