SHISEIDO THE MAKEUP SHEER MATIFYING COMPACT (REFILL)
SMK Matifying Compact SPF 22
FULL PRESCRIBING INFORMATION: CONTENTS*
- SHISEIDO THE MAKEUP SHEER MATIFYING COMPACT (REFILL) Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (B 00)
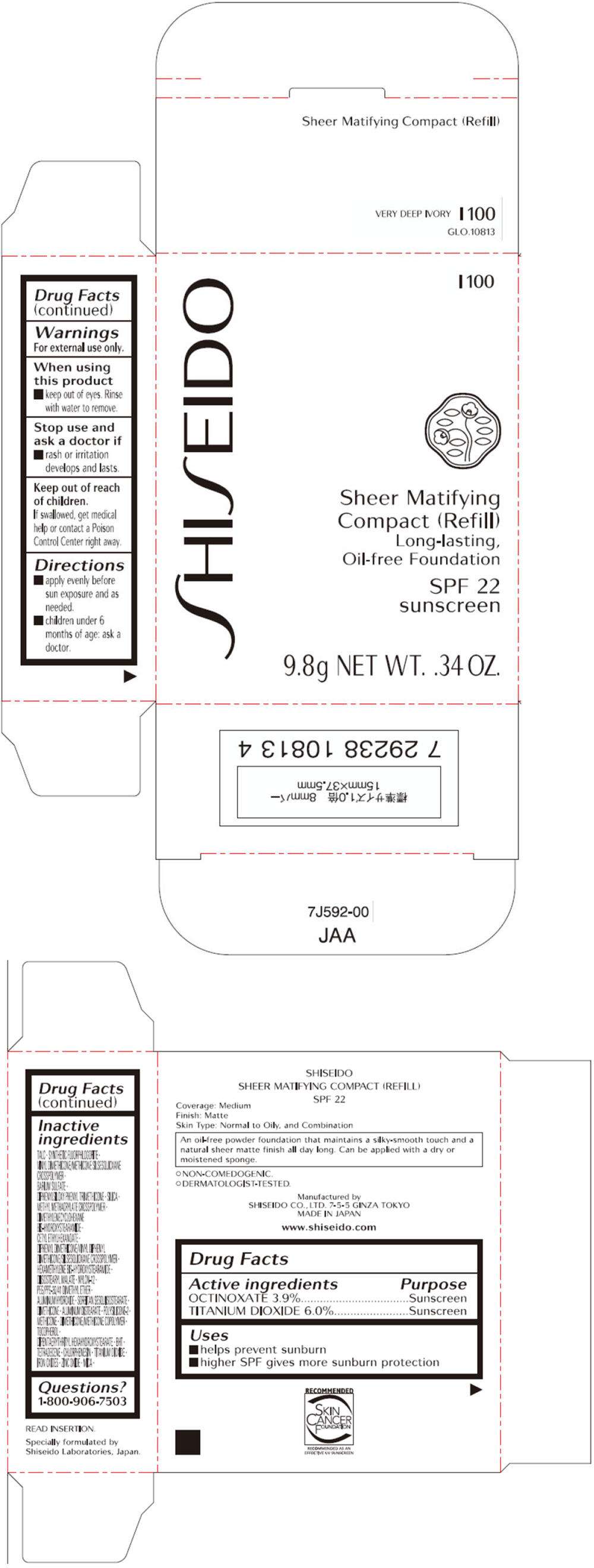
- PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (B 100)
- PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (I 100)
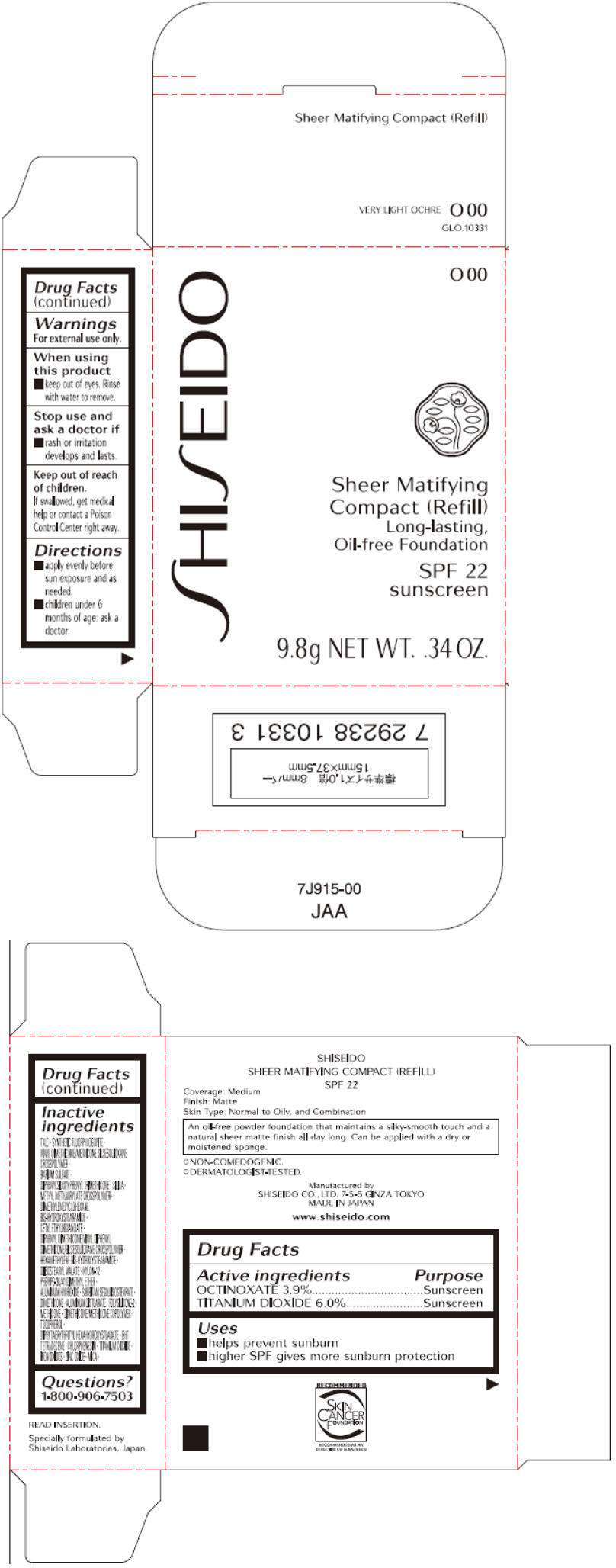
- PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (O 00)
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| OCTINOXATE 3.9% | Sunscreen |
| TITANIUM DIOXIDE 6.0% | Sunscreen |
SHISEIDO THE MAKEUP SHEER MATIFYING COMPACT (REFILL) Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only.
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply evenly before sun exposure and as needed.
- children under 6 months of age: ask a doctor.
Inactive ingredients
TALC
SYNTHETIC FLUORPHLOGOPITE
VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER
BARIUM SULFATE
DIPHENYLSILOXY PHENYL TRIMETHICONE
SILICA
METHYL METHACRYLATE CROSSPOLYMER
DIMETHYLENECYCLOHEXANE BIS-HYDROXYSTEARAMIDE
CETYL ETHYLHEXANOATE
DIPHENYL DIMETHICONE/VINYL DIPHENYL DIMETHICONE/SILSESQUIOXANE CROSSPOLYMER
HEXAMETHYLENE BIS-HYDROXYSTEARAMIDE
DIISOSTEARYL MALATE
NYLON-12
PEG/PPG-36/41 DIMETHYL ETHER
ALUMINUM HYDROXIDE
SORBITAN SESQUIISOSTEARATE
DIMETHICONE
ALUMINUM DISTEARATE
POLYSILICONE-2
METHICONE
DIMETHICONE/METHICONE COPOLYMER
TOCOPHEROL
DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE
BHT
TETRADECENE
CHLORPHENESIN
TITANIUM DIOXIDE
IRON OXIDES
ZINC OXIDE
MICA
Questions?
1-800-906-7503
Manufactured by
SHISEIDO CO., LTD. 7-5-5 GINZA TOKYO
PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (B 00)
SHISEIDO
B 00
Sheer Matifying
Compact (Refill)
Long-lasting,
Oil-free Foundation
SPF 22
sunscreen
9.8g NET WT. .34 OZ.
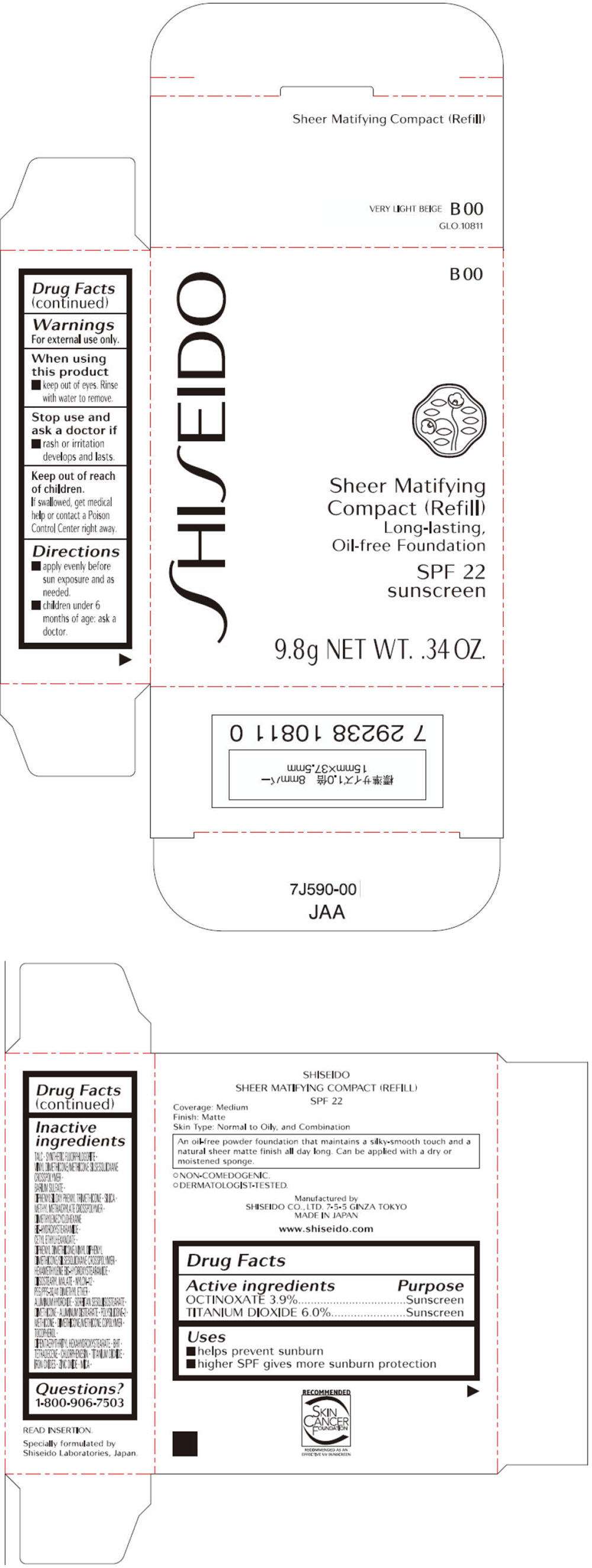
PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (B 100)
SHISEIDO
B 100
Sheer Matifying
Compact (Refill)
Long-lasting,
Oil-free Foundation
SPF 22
sunscreen
9.8g NET WT. .34 OZ.

PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (I 100)
SHISEIDO
I 100
Sheer Matifying
Compact (Refill)
Long-lasting,
Oil-free Foundation
SPF 22
sunscreen
9.8g NET WT. .34 OZ.
PRINCIPAL DISPLAY PANEL - 9.8g Tray Carton (O 00)
SHISEIDO
O 00
Sheer Matifying
Compact (Refill)
Long-lasting,
Oil-free Foundation
SPF 22
sunscreen
9.8g NET WT. .34 OZ.
SHISEIDO THE MAKEUP SHEER MATIFYING COMPACT (REFILL)Octinoxate and Titanium dioxide POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SHISEIDO THE MAKEUP SHEER MATIFYING COMPACT (REFILL)Octinoxate and Titanium dioxide POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SHISEIDO THE MAKEUP SHEER MATIFYING COMPACT (REFILL)Octinoxate and Titanium dioxide POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SHISEIDO THE MAKEUP SHEER MATIFYING COMPACT (REFILL)Octinoxate and Titanium dioxide POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||