SHISEIDO PERFECT HYDRATING BB
SHISEIDO PERFECT HYDRATING BB CREAM
FULL PRESCRIBING INFORMATION: CONTENTS*
- SHISEIDO PERFECT HYDRATING BB Uses
- Warnings
- Directions
- Inactive Ingredients
- SHISEIDO PERFECT HYDRATING BB Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Medium
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Dark
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Light
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| ACTIVE INGREDIENTS: | Purpose |
|---|---|
| ENSULIZOLE 2.0% | Sunscreen |
| OCTINOXATE 7.4% | Sunscreen |
| TITANIUM DIOXIDE 7.2% | Sunscreen |
SHISEIDO PERFECT HYDRATING BB Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions ), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
WATER, DIMETHICONE, SD ALCOHOL 40-B, PENTAERYTHRITYL TETRAETHYLHEXANOATE, PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, POLYMETHYL METHACRYLATE, GLYCERIN, DIPROPYLENE GLYCOL, DISTEARDIMONIUM HECTORITE, HYDROGENATED POLYDECENE, TRIMETHYLSILOXYSILICATE, SILICA, XYLITOL, PEG-6, PEG-32, PEG/PPG-14/7 DIMETHYL ETHER, THIOTAURINE, SAXIFRAGA SARMENTOSA EXTRACT, ARGININE HCl, ONONIS SPINOSA ROOT EXTRACT, SODIUM PCA, SOPHORA ANGUSTIFOLIA ROOT EXTRACT, PPG-17, ALUMINUM HYDROXIDE, AMINOMETHYL PROPANOL, ALUMINUM DISTEARATE, ISOSTEARIC ACID, STEARIC ACID, TRISODIUM EDTA, POLYMETHYLSILSESQUIOXANE, TALC, BUTYLENE GLYCOL, ALCOHOL, ALUMINA, POLYSILICONE-2, TRIETHOXYCAPRYLYLSILANE, POLYBUTYLENE GLYCOL/PPG-9/1 COPOLYMER, BHT, SYZYGIUM JAMBOS LEAF EXTRACT, TOCOPHEROL, PHENOXYETHANOL, FRAGRANCE, IRON OXIDES, TITANIUM DIOXIDE,
SHISEIDO PERFECT HYDRATING BB Other information
- protect this product in this container from excessive heat and direct sun.
Questions or comments?
Call toll free 1-800-906-7503
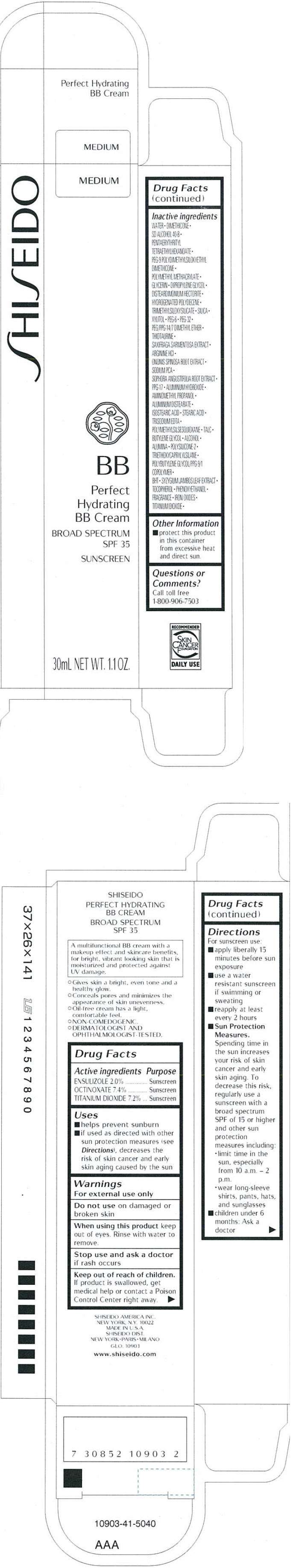
PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Medium
S HI S EIDO
MEDIUM
BB
Perfect
Hydrating
BB Cream
BROAD SPECTRUM
SPF 35
SUNSCREEN
30mL NET WT. 1.1 OZ.
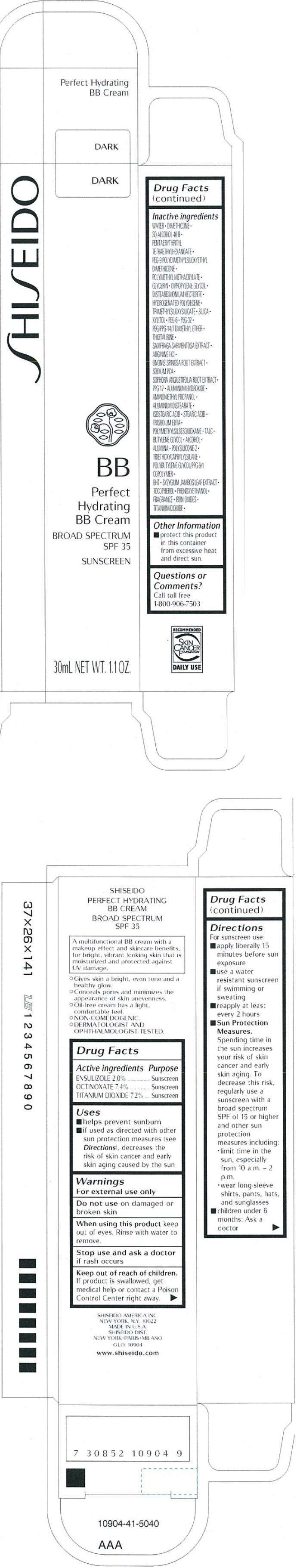
PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Dark
S HI S EIDO
DARK
BB
Perfect
Hydrating
BB Cream
BROAD SPECTRUM
SPF 35
SUNSCREEN
30mL NET WT. 1.1 OZ.
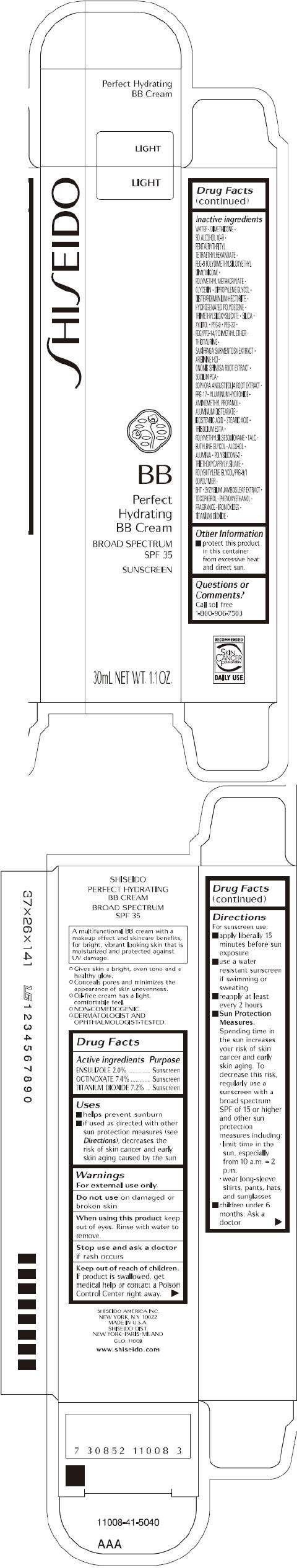
PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Light
S HI S EIDO
LIGHT
BB
Perfect
Hydrating
BB Cream
BROAD SPECTRUM
SPF 35
SUNSCREEN
30mL NET WT. 1.1 OZ.
SHISEIDO PERFECT HYDRATING BBENSULIZOLE, OCTINOXATE, and TITANIUM DIOXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SHISEIDO PERFECT HYDRATING BBENSULIZOLE, OCTINOXATE, and TITANIUM DIOXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||