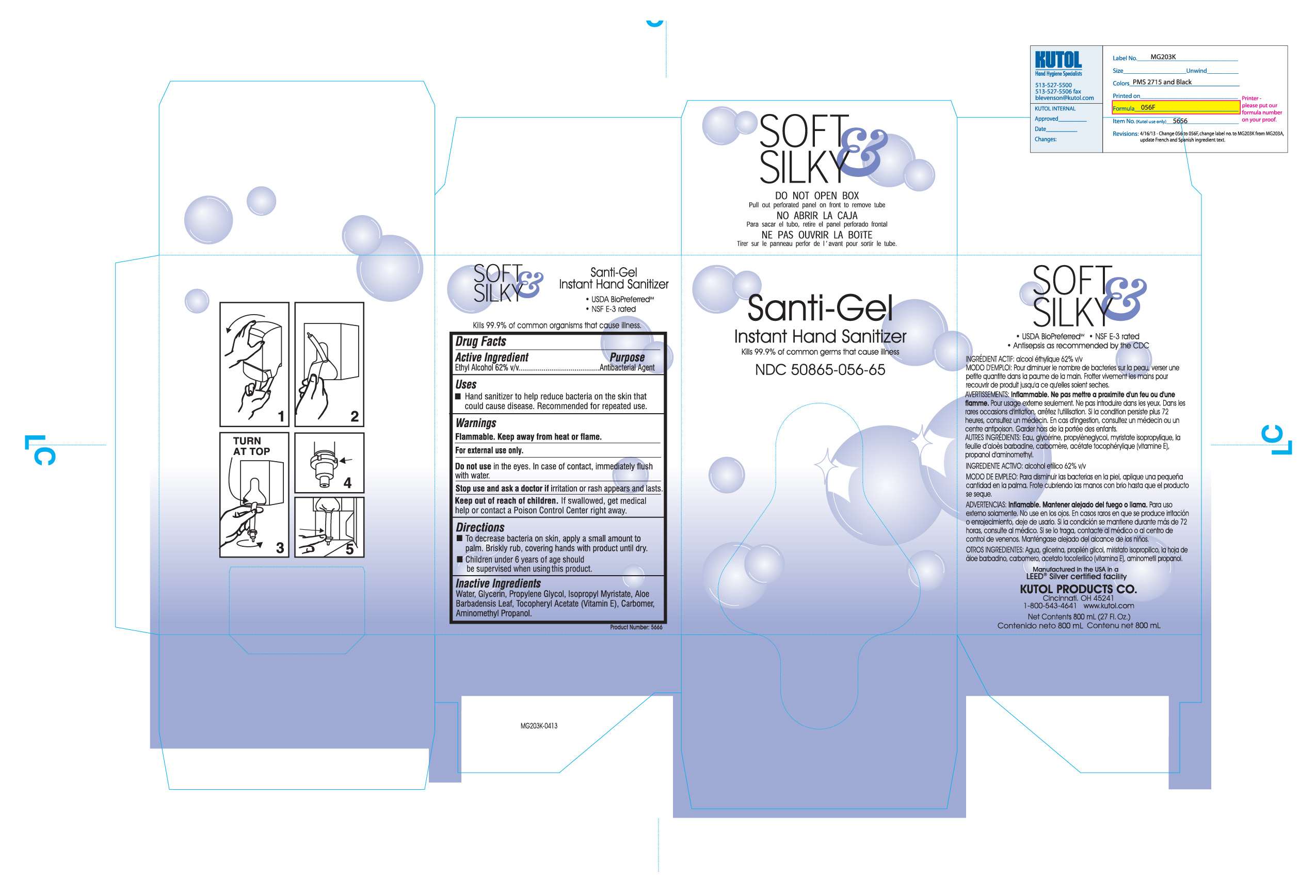
Santi-Gel Instant Hand Sanitizer
Kutol Products Company, Inc.
Kutol Products Company, Inc.
Santi-Gel Instant Hand Sanitizer
FULL PRESCRIBING INFORMATION
Flammable. Keep away from heat or flame.
For external use only.
Do not use in the eyes. In case of contact, immediately flush with water.
Stop use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Active ingredient
Ethyl Alcohol 62% v/v.........Antibacterial Agent
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Water, Glycerin, Propylene Glycol, Isopropyl Mryistate, Aloe Barbadensis leaf, Tocopheryl Acetate (Vitamin E), Carbomer, Aminomethyl Propanol
To decrease bacteria on skin, apply a small amount to palm. Briskly rub, covering hands with product until dry.
Children under 6 years of age should be supervised when using this product.
Purpose
Hand Sanitizer to help reduce bacteria on the skin that could cause disease. Recommended for repeated use.
Uses
Hand Sanitizer to help reduce bacteria on the skin that could cause disease. Recommended for repeated use.
Do not use in the eyes. In case of contact, immediately flush with water.
Stop use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
 50865-056-65.jpg
50865-056-65.jpg
Santi-Gel Instant Hand SanitizerSanti-Gel Instant Hand Sanitizer SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||