REZIL
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS SECTION
- PURPOSE SECTION
- KEEP OUT OF REACH OF CHILDREN SECTION
- INDICATIONS AND USES SECTION
- WARNINGS SECTION
- REZIL DOSAGE AND ADMINISTRATION SECTION
- INACTIVE INGREDIENTS SECTION
- QUESTIONS OR COMMENTS SECTION
- OTHER INFORMATION SECTION
- PACKAGE LABEL SECTION
FULL PRESCRIBING INFORMATION
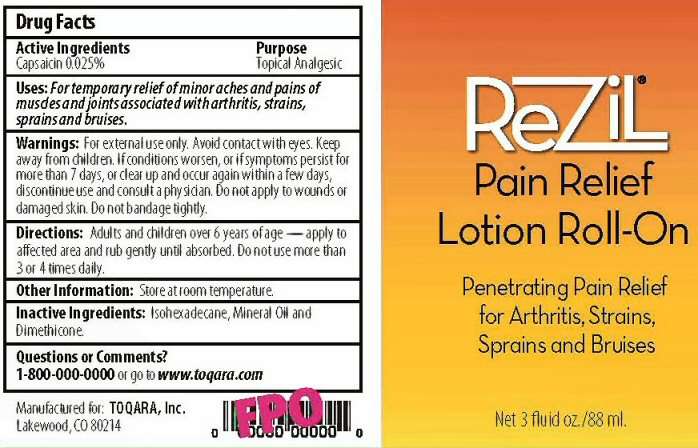
ACTIVE INGREDIENTS SECTION
DRUG FACTS
CAPSAICIN 0.025%
PURPOSE SECTION
TOPICAL ANALGESIC
KEEP OUT OF REACH OF CHILDREN SECTION
KEEP AWAY FROM CHILDREN.
INDICATIONS AND USES SECTION
USES: FOR TEMPORARY RELIEF OF MINOR ACHES, AND PAINS OF MUSCLES AND JOINTS ASSOCIATED WITH ARTHRITIS, STRAINS, SPRAINS, AND BRUISES.
WARNINGS SECTION
WARNINGS: FOR EXTERNAL USE ONLY. AVOID CONTACT WITH EYES. IF CONDITION WORSEN, OR IF SYMPTOMS PERSIST FOR MORE THAN 7 DAYS, OR CLEAR UP AND OCCUR AGAIN WITHIN A FEW DAYS, DISCONTINUE USE A CONSULT A PHYSICIAN. DO NOT APPLY TO WOUNDS OR DAMAGED SKIN. DO NOT BANDAGE TIGHTLY.
DOSAGE AND ADMINISTRATION SECTION
DIRECTIONS: ADULTS AND CHILDREN OVER 6 YEARS OF AGE - APPLY TO AFFECTED AREA AND RUB GENTLY UNTIL ABSORBED. DO NOT USE MORE THAN 3 OR 4 TIMES DAILY.
INACTIVE INGREDIENTS SECTION
ISOHEXADECANE, MINERAL OIL AND DIMETHICONE
QUESTIONS OR COMMENTS SECTION
QUESTIONS OR COMMENTS? 1-800-000-0000 OR GO TO WWW.TOQARA.COM
OTHER INFORMATION SECTION
OTHER INFORMATION: STORE AT ROOM TEMPERATURE
PACKAGE LABEL SECTION
REZIL PAIN RELIEF LOTION ROLL-ON
PENETRATING PAIN RELIEF FOR ARTHRITIS, STRAINS, SPRAINS AND BRUISES
NET 3 FLUID OZ./88ML MANUFACTURED FOR TOQARA, INC. LAKEWOOD, CO 80214



REZILCAPSAICIN LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||