Reboost
Reboost Tablet
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
- PURPOSE
- REBOOST DOSAGE AND ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- REBOOST INDICATIONS AND USAGE
FULL PRESCRIBING INFORMATION
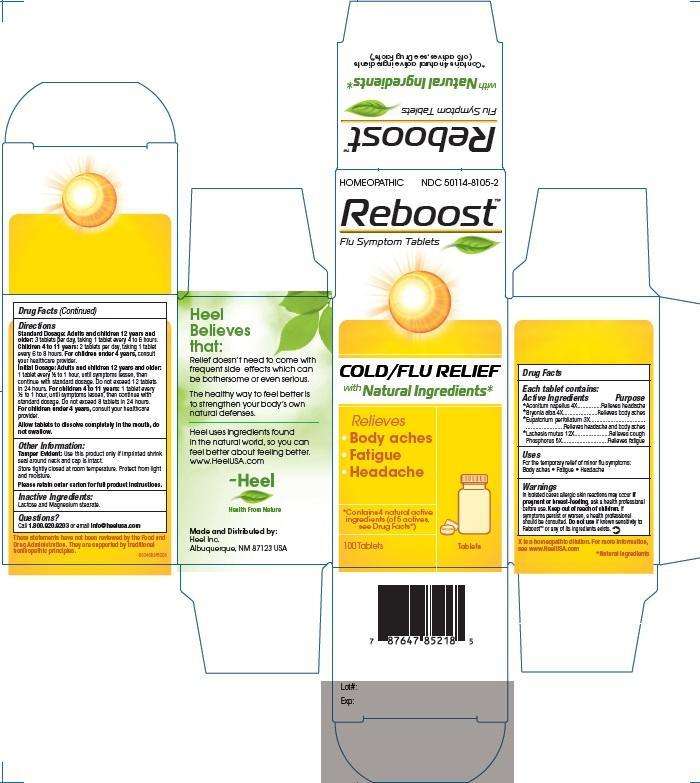
ACTIVE INGREDIENTS
Active Ingredients: Each tablet contains: Aconitum napellus 4X, Bryonia alba 4X, Eupatorium perfollatum 3X, Lachesis mutus 12X, Phosphorus 5X
INACTIVE INGREDIENTS
Inactive Ingredients: Lactose and Magnessium stearate
PURPOSE
Aconitum napellus 4X................Relieves headache
Bryonia alba 4X.........................Relieves body aches
Eupatorium perfollatum 3X.......Relieves headache and body aches
Lachesis mutus 12X..................Relieves cough
Phosphorus 5X..........................Relieves fatigue
REBOOST DOSAGE AND ADMINISTRATION
Standard Dosage: Adults and children 12 years and older: 3 tablets per day, taking 1 tablet every 4 to 6 hours.
Children 4 to 11 years: 2 tablets per day, taking 1 tablet every 6 to 8 hours.
For children under 4 years, consult your healthcare provider.
Initial Dosage: Adults and children 12 years and older: 1 tablet every 1/2 to 1 hour, until symptoms lessen, then continue with standard dosage. Do not exceed 12 tablets in 24 hours.
Children 4 to 11 years: 1 tablet every 1/2 to 1 hour, until symptoms lessen, then continue with standard dosage. Do not exceed 8 tablets in 24 hours.
For children under 4 years, consult your healthcare provider.
Allow tablets to dissolve completely in mouth, do not swallow.
WARNINGS
In isolated cases allergic skin reactions may occur. If pregnant or breast-feeding, ask a healthcare provider before use. Keep out of reach of children. If symptoms persist or worsen, a healthcare provider should be consulted. Do not use if known sensitivity to Reboost or any of its ingredients exists.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.
REBOOST INDICATIONS AND USAGE
For the temporary relief of minor flu symptoms:
- Body aches
- Fatigue
- Headache
ReboostACONITUM NAPELLUS and BRYONIA ALBA ROOT and EUPATORIUM PERFOLIATUM FLOWERING TOP and LACHESIS MUTA VENOM and PHOSPHORUS TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||