Quflora Pediatric
CarWin Pharmaceutical Associates, LLC
Quflora™ Pediatric Chewable Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- QUFLORA PEDIATRIC DESCRIPTION
- CLINICAL PHARMACOLOGY
- QUFLORA PEDIATRIC INDICATIONS AND USAGE
- QUFLORA PEDIATRIC CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- QUFLORA PEDIATRIC ADVERSE REACTIONS
- OVERDOSAGE
- QUFLORA PEDIATRIC DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 0.25 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 1.0 mg Tablet Bottle Label
FULL PRESCRIBING INFORMATION
| Nutrition Facts Serving Size: 1 Chewable Tablet |
||
|---|---|---|
| Amount Per Tablet | % DV for Adults and Children 4 or more years of age | |
| Vitamin A (as Acetate) | 1,200 IU | 24% |
| Vitamin C (as Ascorbic Acid) | 60 mg | 100% |
| Vitamin D3 (as Cholecalciferol) | 400 IU | 100% |
| Vitamin E (as DL-Alpha-Tocopheryl Acetate) | 15 IU | 50% |
| Thiamin (as Thiamine HCl, Vitamin B1) | 1.2 mg | 80% |
| Riboflavin (Vitamin B2) | 1.3 mg | 76% |
| Niacin (as Niacinamide) | 5 mg | 25% |
| Vitamin B6 (as Pyridoxine HCl) | 1.5 mg | 75% |
Folate (as 200 mcg Quatrefolic® ((6S-5-methyltetrahydrofolic acid, glucosamine salt |
208 mcg | 52% |
| Vitamin B12 (as Cyanocobalamin) | 4 mcg | 67% |
| Magnesium (as Magnesium Oxide) | 15 mg | 4% |
| Copper (as Cupric Sulfate) | 1 mg | 50% |
| Fluoride (as Sodium Fluoride) | 0.5 mg |
 |
Active Ingredient for caries prophylaxis: Fluoride as Sodium Fluoride (NaF).
Other Ingredients:
Artificial grape flavor, citric acid, fumed silica, magnesium stearate, microcrystalline cellulose, silicon dioxide, sucralose, sucrose, and stearic acid.
QUFLORA PEDIATRIC DESCRIPTION
Quflora™ Pediatric Chewable Tablets are for use as a dental caries preventative in children.
Each Quflora™ 1.0 mg F
Each Quflora™ 0.5 mg F
Each Quflora™ 0.25 mg F
CLINICAL PHARMACOLOGY
It is well established that fluoridation of the water supply (1 ppm fluoride) during the period of tooth development leads to a significant decrease in the incidence of dental caries.
Quflora™ Pediatric Chewable Tablets provide sodium fluoride and twelve essential vitamins and minerals in a chewable tablet. Because the tablets are chewable, they provide a topical as well as systemic source of fluoride.
Hydroxyapatite is the principal crystal for all calcified tissue in the human body. The fluoride ion reacts with the hydroxyapatite in the tooth as it is formed to produce the more caries-resistant crystal, fluorapatite. The reaction may be expressed by the equation:
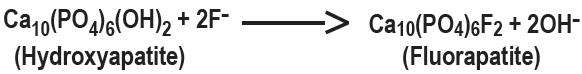
Three stages of fluoride deposition in tooth enamel can be distinguished:
- Small amounts (reflecting the low levels of fluoride in tissue fluids) are incorporated into the enamel crystals while they are being formed.
- After enamel has been laid down, fluoride deposition continues in the surface enamel. Diffusion of fluoride from the surface inward is apparently restricted.
- After eruption, the surface enamel acquires fluoride from the water, food, supplementary fluoride and smaller amounts from saliva.
QUFLORA PEDIATRIC INDICATIONS AND USAGE
Supplementation of the diet with twelve essential vitamins and minerals.
Supplementation of the diet with fluoride for caries prophylaxis.
See Dosage and Administration for complete dosing guidelines.
Quflora™ Pediatric Chewable Tablets supply significant amounts of Vitamins A, C, D, E, Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, along with Magnesium, and Copper to supplement the diet, and to help assure that nutritional deficiencies of these vitamins and minerals will not develop. Thus, in a single easy-to-use preparation, children obtain twelve essential vitamins and minerals, plus fluoride.
The American Academy of Pediatrics recommends that children up to age 16, in areas where drinking water contains less than optimal levels of fluoride, receive daily fluoride supplementation.
Children using Quflora™ Pediatric Chewable Tablets regularly should receive semiannual dental examinations. The regular brushing of teeth and attention to good oral hygiene practices are also essential.
QUFLORA PEDIATRIC CONTRAINDICATIONS
Do not use Quflora™ Pediatric Chewable Tablets in areas where the fluoride content of the drinking water exceeds 0.6 ppm F-. Chronic renal insufficiency and failure, arthralgia, gastrointestinal ulceration and osteomalacia are contraindications to fluoride therapy. Quflora™ Pediatric Chewable Tablets (any strength) are not indicated for use in adults.
WARNINGS
Prolonged daily ingestion of quantities greater than the recommended amount may result in various degrees of dental fluorosis in pediatric patients under 6 years of age, especially if the water fluoridation exceeds 0.6 ppm.
This product should be chewed and is not recommended for children under age 4 due to risk of choking.
Read directions carefully before using. Keep out of reach of infants and children.
PRECAUTIONS
General
Please refer to the CONTRAINDICATIONS , WARNINGS and OVERDOSAGE sections for overdosage concerns.
The suggested dose of Quflora™ Pediatric Chewable Tablets should not be exceeded, since dental fluorosis may result from continued ingestion of large amounts of fluoride.
Before prescribing Quflora™ Pediatric Chewable Tablets, read these important considerations when using the dosage schedule found in Dosage and Administration.
- If fluoride level is unknown, drinking water should be tested for fluoride content before supplements are prescribed. For testing of fluoride content, contact the local or state health department.
- All sources of fluoride should be evaluated with a thorough fluoride history.
- Patient exposure to multiple water sources can make proper prescribing complex.
- Ingestion of higher than recommended levels of fluoride by children has been associated with an increase in mild dental fluorosis in developing, unerupted teeth.
- Fluoride supplements require long-term compliance on a daily basis.
Drug Interactions
Do not eat or drink dairy products within one hour of fluoride administration. Incompatibility of fluoride with dairy foods has been reported due to formation of calcium fluoride which is poorly absorbed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a study conducted in rodents, no carcinogenesis was found in male and female mice and female rats treated with fluoride at dose levels ranging from 4.1 to 9.1 mg/kg of body weight. Equivocal evidence of carcinogenesis was reported for male rats treated with 2.5 and 4.1 mg/kg of body weight. In a second study, no carcinogenesis was observed in rats, males or females, treated with fluoride up to 11.3 mg/kg of body weight. This dose is at least 400 times greater than the recommended daily dose of Quflora™ Pediatric Chewable Tablets. Fluoride ion is not mutagenic in standard bacterial systems. It has been shown that fluoride ion has potential to induce chromosome aberrations in cultured human and rodent cells at doses much higher than those to which humans are exposed. In vivo data is conflicting. Some studies report chromosome damage in rodents while other studies using similar protocols report negative results. Potential adverse reproductive effects of fluoride exposure in humans have not been adequately evaluated. Adverse effects on reproduction were reported for rats, mice, fox, and cattle exposed to 100 ppm or greater concentrations of fluoride in their diet or drinking water. Other studies conducted in rats demonstrated that lower doses of fluoride (5 mg/kg of body weight) did not result in impaired fertility and reproductive capabilities. This dose is approximately 200 times greater than the recommended daily dose of Quflora™ Pediatric Chewable Tablets.
Pregnancy
Teratogenic Effects
Pregnancy Category B
It has been shown that fluoride crosses the placenta of rats, but only 0.01% of the amount administered is incorporated in fetal tissue. Animal studies (rats, mice, rabbits) have shown that fluoride is not a teratogen. Maternal exposure to 12.2 mg fluoride/kg of body weight (rats) or 13.1 mg/kg of body weight (rabbits) did not affect the litter size or fetal weight and did not increase the frequency of skeletal or visceral malformations. Epidemiological studies conducted in areas with high levels of naturally fluoridated water showed no increase in birth defects. Heavy exposure to fluoride during in utero development may result in skeletal fluorosis which becomes evident in childhood.
Nursing Mothers
It is not known if fluoride is excreted in human milk. However, many drugs are excreted in human milk and caution should be exercised when Quflora™ Pediatric Chewable Tablets are administered to a nursing woman. Reduced milk production was reported in farm-raised fox when the animals were fed a diet containing a high concentration of fluoride (98-137 mg/kg of body weight). No adverse effects on parturition, lactation, or offspring were seen in rats administered fluoride up to 5 mg/kg of body weight. This dose is at least 200 times greater than the recommended daily dose of Quflora™ Pediatric Chewable Tablets. Quflora™ Pediatric Chewable Tablets (any strength) are not indicated for use in adults.
Pediatric Use
The use of Quflora™ Pediatric Chewable Tablets as a caries preventive in pediatric age groups 6 months to 16 years is supported by evidence from adequate and well controlled studies on fluoride supplementation from birth through adolescence.
Geriatric Use
Quflora™ Pediatric Chewable Tablets (any strength) are not indicated for use in geriatric patients.
QUFLORA PEDIATRIC ADVERSE REACTIONS
Allergic rash and other idiosyncrasies have been rarely reported.
Call your doctor for medical advice about side effects. You may report adverse side effects or obtain product information by contacting CarWin Pharmaceutical Associates, LLC at 1-866-633-9033
OVERDOSAGE
Accidental ingestion of large amounts of fluoride may result in acute burning in the mouth and sore tongue. Nausea, vomiting, and diarrhea may occur soon after ingestion (within 30 minutes) and are accompanied by salivation, hematemesis, and epigastric cramping abdominal pain. These symptoms may persist for 24 hours. If less than 5 mg fluoride/kg body weight (i.e., less than 2.3 mg fluoride/lb body weight) has been ingested, give calcium (e.g., milk) orally to relieve gastrointestinal symptoms and observe for a few hours. If more than 5 mg fluoride/kg body weight (i.e., more than 2.3 mg fluoride/lb body weight) has been ingested, induce vomiting, give orally soluble calcium (e.g., milk, 5% calcium gluconate or calcium lactate solution) and immediately seek medical assistance. For accidental ingestion of more than 15 mg fluoride/kg of body weight (i.e., more than 6.9 mg fluoride/lb body weight), induce vomiting and admit immediately to a hospital facility.
A treatment dose of Quflora™ Pediatric Chewable Tablets contains 0.25, 0.5 or 1 mg of fluoride. The treatment of choice depends upon the age of the child and the water fluoride content. A bottle of 30 0.25 mg tablets contains 7.5 mg fluoride. A bottle of 30 0.5 mg tablets contains 15 mg fluoride. A bottle of 30 1 mg tablets contains 30 mg fluoride. The total amount of sodium fluoride in a bottle of 30 Quflora™ Pediatric Chewable Tablets (all strengths) conforms with the recommendations of the American Dental Association for the maximum to be dispensed at one time for safety purposes.
QUFLORA PEDIATRIC DOSAGE AND ADMINISTRATION
Dissolve in the mouth or chew before swallowing once daily, preferably at bedtime after brushing teeth. See schedule below to determine dosage.
| Age | Less than 0.3ppm | 0.3-0.6 ppm | Greater than 0.6 ppm |
|---|---|---|---|
| Birth-6 months | None | None | None |
| 6 months-3 years | 0.25 mg/day |
None | None |
| 3-6 years | 0.50 mg/day |
0.25 mg/day | None |
| 6-16 years | 1.0 mg/day | 0.50 mg/day | None |
Dosing schedule approved by the American Dental Association, American Academy of Pediatrics, American Academy of Pediatric Dentistry
HOW SUPPLIED
Quflora™ 1.0 mg F (full strength) Pediatric Chewable Tablets are available as white chewable tablets imprinted with "105" in bottles of 30 (NDC 15370-105-30) and 1ct physician samples (NDC 15370-105-99), Grape flavor.
Quflora™ 0.5 mg F (half strength) Pediatric Chewable Tablets are available as white chewable tablets imprinted with "104" in bottles of 30 (NDC 15370-104-30) and 1ct physician samples (NDC 15370-104-99), Grape flavor.
Quflora™ 0.25 mg F (quarter strength) Pediatric Chewable Tablets are available as white chewable tablets imprinted with "103" in bottles of 30 (NDC 15370-103-30) and 1ct physician samples (NDC 15370-103-99), Grape flavor.
STORAGE
Store at Controlled Room Temperature, 20-25°C (68-77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature].
Protect from light and moisture. Dispense in a tight,light-resistant container with a child-resistant closure as defined in the USP/NF.
Rx only
Manufactured in Canada
Manufactured for:
CarWin Pharmaceutical Associates, LLC
Slidell, LA 70461
CW-104-1
REV 1/14
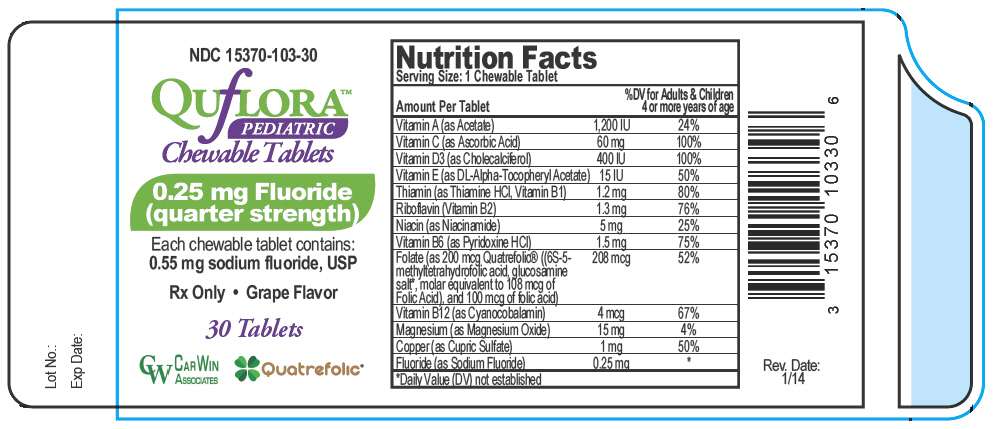
PRINCIPAL DISPLAY PANEL - 0.25 mg Tablet Bottle Label
NDC 15370-103-30
QUfLORA™
PEDIATRIC
Chewable Tablets
0.25 mg Fluoride
(quarter strength)
Each chewable tablet contains:
0.55 mg sodium fluoride, USP
Rx Only • Grape Flavor
30 Tablets
CARWIN
ASSOCIATES
Quatrefolic®
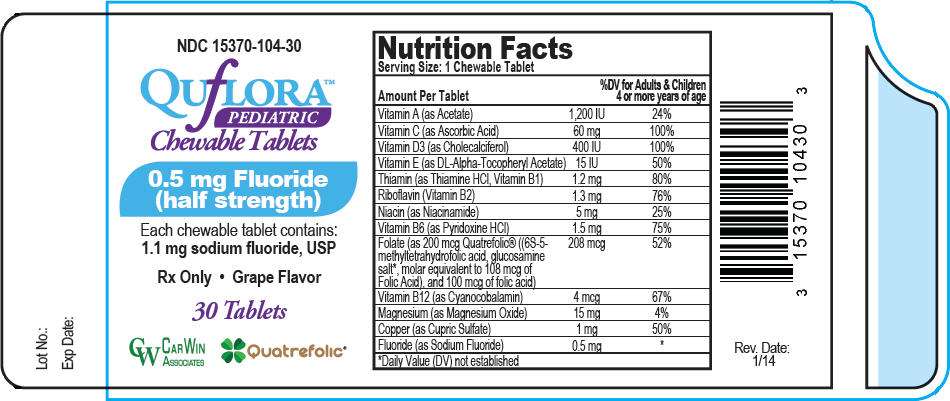
PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Bottle Label
NDC 15370-104-30
QUfLORA™
PEDIATRIC
Chewable Tablets
0.5 mg Fluoride
(half strength)
Each chewable tablet contains:
1.1 mg sodium fluoride, USP
Rx Only • Grape Flavor
30 Tablets
CARWIN
ASSOCIATES
Quatrefolic®
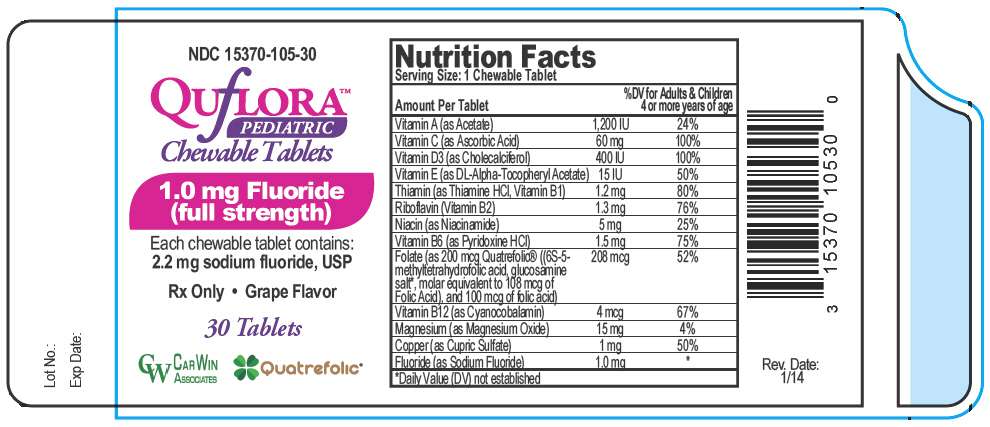
PRINCIPAL DISPLAY PANEL - 1.0 mg Tablet Bottle Label
NDC 15370-105-30
QUfLORA™
PEDIATRIC
Chewable Tablets
1.0 mg Fluoride
(full strength)
Each chewable tablet contains:
2.2 mg sodium fluoride, USP
Rx Only • Grape Flavor
30 Tablets
CARWIN
ASSOCIATES
Quatrefolic®
Quflora PediatricVITAMIN A ACETATE, ASCORBIC ACID, CHOLECALCIFEROL, .ALPHA.-TOCOPHEROL, DL-, THIAMINE HYDROCHLORIDE, RIBOFLAVIN, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, LEVOMEFOLIC ACID, FOLIC ACID, CYANOCOBALAMIN, MAGNESIUM OXIDE, CUPRIC SULFATE, and SODIUM FLUORIDE TABLET, CHEWABLE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quflora PediatricVITAMIN A ACETATE, ASCORBIC ACID, CHOLECALCIFEROL, .ALPHA.-TOCOPHEROL, DL-, THIAMINE HYDROCHLORIDE, RIBOFLAVIN, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, LEVOMEFOLIC ACID, FOLIC ACID, CYANOCOBALAMIN, MAGNESIUM OXIDE, CUPRIC SULFATE, and SODIUM FLUORIDE TABLET, CHEWABLE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quflora PediatricVITAMIN A ACETATE, ASCORBIC ACID, CHOLECALCIFEROL, .ALPHA.-TOCOPHEROL, DL-, THIAMINE HYDROCHLORIDE, RIBOFLAVIN, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, LEVOMEFOLIC ACID, FOLIC ACID, CYANOCOBALAMIN, MAGNESIUM OXIDE, CUPRIC SULFATE, and SODIUM FLUORIDE TABLET, CHEWABLE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||