Promolaxin
Dispensing Solutions, Inc.
PSS World Medical, Inc.
Promolaxin™ Docusate Sodium Stool Softener
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Promolaxin Uses
- Warnings
- Directions
- Inactive ingredients
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
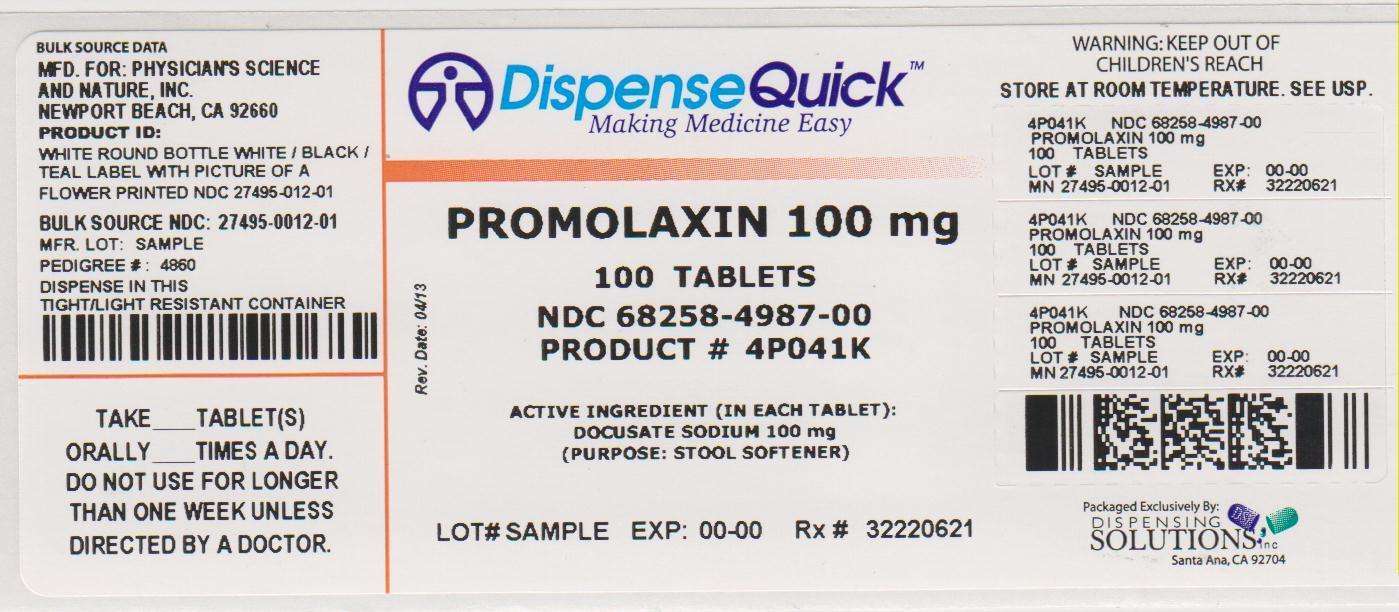
Active ingredient
Docusate Sodium 100 mg
Purpose
Stool Softener
Promolaxin Uses
- for relief of occasional constipation (irregularity). This product generally produces a bowel movement within 12 to 72 hours.
Warnings
- laxative products for longer than one week unless directed to do so by a doctor
- if you are presently taking mineral oil unless told to do so by a doctor
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- you have rectal bleeding
- you fail to have a bowel movement after use
These could be signs of a serious condition.
ask a doctor before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and older:
Take 1 tablet as needed, not to exceed more than 3 tablets daily, or as directed by a doctor.
Children under 12 years of age:
Consult a doctor before use.
- Each tablet contains: Calcium 40 mg
- Each tablet contains: Sodium 10 mg
- Store at room temperature.
- Do not use if imprinted safety seal is broken or missing.
Inactive ingredients
Croscarmellose Sodium, Dicalcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Pregelatinized Starch, Silica, Sodium Benzoate, Stearic Acid.
If you have any questions or comments, or to report an adverse event,
please contact 714-875-6316.
Manufactured for: Physician's Science and Nature, Inc.
220 Newport Center Drive 11-634, Newport Beach, CA 92660
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 68258-4987-00
PromolaxinDocusate sodium TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||