Proactiv
Proactiv Solution Concealer Plus
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Directions
- Warnings
- Inactive Ingredients
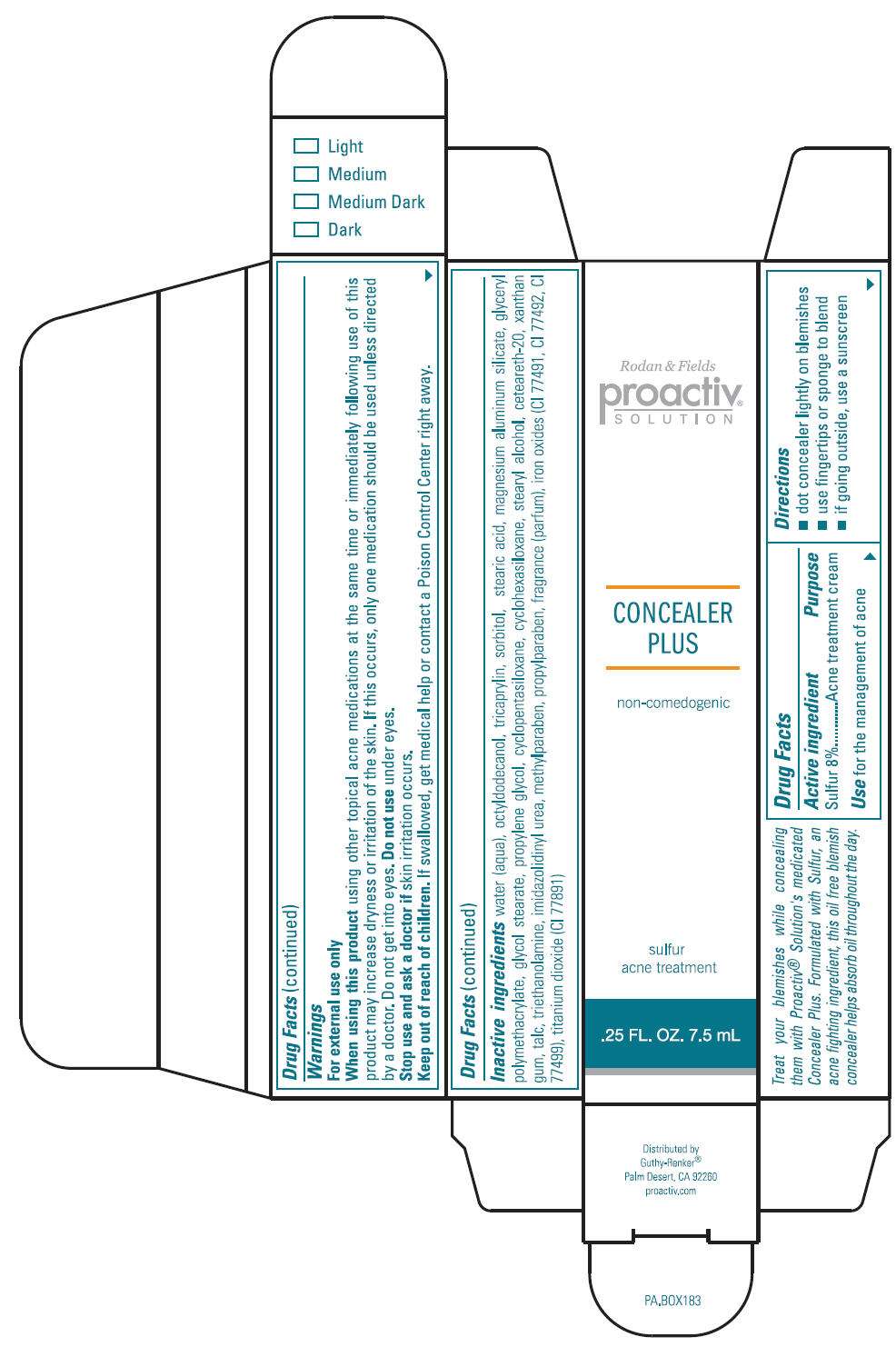
- PRINCIPAL DISPLAY PANEL - 7.5 mL Vial Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Sulfur 8%
Purpose
Acne Treatment Cream
Use
For the management of acne
Directions
- dot concealer lightly on blemishes
- use fingertips or sponge to blend
- if going outside, use a sunscreen
Warnings
For external use only
When using this product
using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor. Do not get into eyes.
Do not use under eyes.
Stop use and ask a doctor if skin irritation occurs.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
water (aqua), octyldodecanol, tricaprylin, sorbitol, stearic acid, magnesium aluminum silicate, glyceryl polymethacrylate, glycol stearate, propylene glycol, cyclopentasiloxane, cyclohexasiloxane, stearyl alcohol, ceteareth-20, xanthan gum, talc, triethanolamine, imidazolidinyl urea, methylparaben, propylparaben, fragrance (parfum), iron oxides (CI 77491, CI 77492, CI 77499), titanium dioxide (CI 77891)
PRINCIPAL DISPLAY PANEL - 7.5 mL Vial Carton
Rodan & Fields
proactiv
®
SOLUTION
CONCEALER
PLUS
non-comedogenic
sulfur
acne treatment
.25 FL. OZ. 7.5 mL
ProactivSULFUR LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||