Prednisone
FULL PRESCRIBING INFORMATION: CONTENTS*
- PREDNISONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PREDNISONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- INFORMATION FOR PATIENTS
- PREDNISONE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PREDNISONE DESCRIPTION
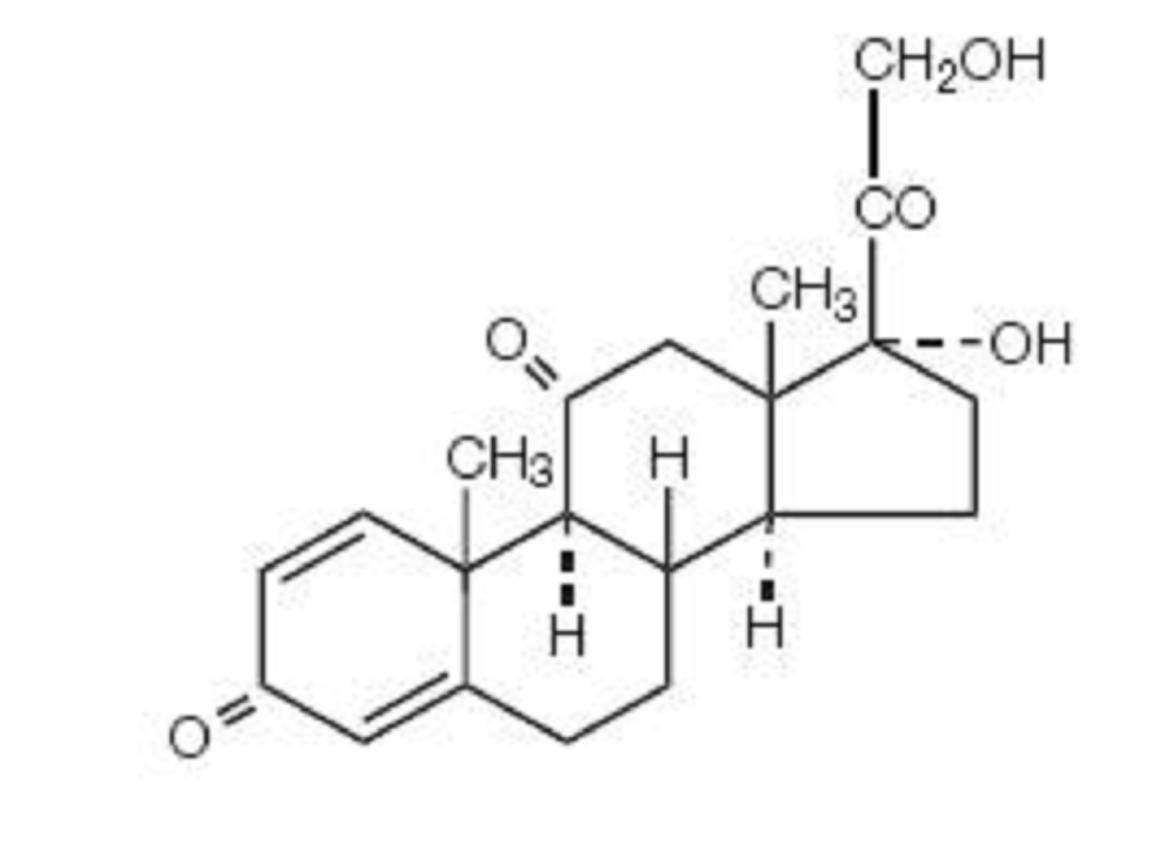
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
Endocrine disorders:
Rheumatic disorders:
Collagen diseases:
Dermatologic diseases:
Allergic states:
Ophthalmic diseases:
Respiratory diseases:
Hematologic disorders:
Neoplastic diseases:
Edematous states:
Gastrointestinal diseases:
Nervous system:
Miscellaneous:
PREDNISONE CONTRAINDICATIONS
WARNINGS
Usage in pregnancy
PRECAUTIONS
General PrecautionsDOSAGE AND ADMINISTRATION.
DRUG INTERACTIONS
INFORMATION FOR PATIENTS
PREDNISONE ADVERSE REACTIONS
Fluid and electrolyte disturbances:Musculoskeletal:
Gastrointestinal:
Dermatologic:
Metabolic:
Neurological:
Endocrine:
Ophthalmic:
Additional Reactions:
DOSAGE & ADMINISTRATION
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT.Multiple Sclerosis
Alternate Day Therapy
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PrednisonePrednisone TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!