Prednisone
FULL PRESCRIBING INFORMATION: CONTENTS*
- PREDNISONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PREDNISONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PREDNISONE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PREDNISONE DESCRIPTION
Inactive Ingredients
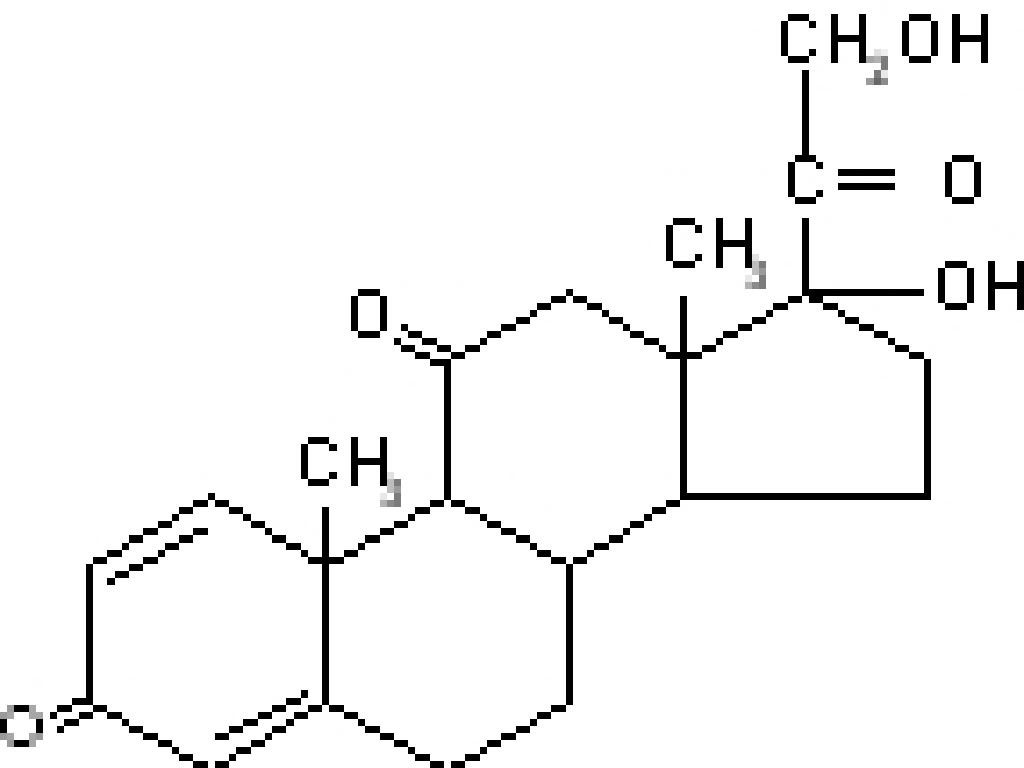
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
Endocrine Disorders
Rheumatic Disorders
Collagen Diseases
Dermatologic Diseases
Allergic States
Ophthalmic Diseases
Respiratory Diseases
Hematologic Disorders
Neoplastic Diseases
Edematous States
Gastrointestinal Diseases
Miscellaneous
PREDNISONE CONTRAINDICATIONS
WARNINGS
GeneralADVERSE REACTIONSAllergic Reactions
Cardio-Renal
Endocrine
Infection
General
Fungal Infections
PRECAUTIONSDrug InteractionsAmphotericin B Injection and Potassium-Depleting Agents
Special Pathogens
Tuberculosis
Vaccination
Viral Infections
Ophthalmic
PRECAUTIONS
General PrecautionsCardio-Renal
Endocrine
Gastrointestinal
Musculoskeletal
Neuro-Psychiatric
DOSAGE AND ADMINISTRATIONMultiple Sclerosis
Ophthalmic
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
Amphotericin B Injection and Potassium-Depleting AgentsAntibiotics
PRECAUTIONSDrug InteractionsHepatic Enzyme Inducers, Inhibitors and Substrates
Anticholinesterases
Anticoagulants, Oral
Antidiabetics
Antitubercular drugs
Bupropion
Cholestyramine
Cyclosporine
Digitalis Glycosides
Estrogens, Including Oral Contraceptives
Fluoroquinolones
Hepatic Enzyme Inducers, Inhibitors and Substrates
Ketoconazole
Nonsteroidal Anti-Inflammatory Agents (NSAIDS)
Phenytoin
Quetiapine
Skin Tests
Thalidomide
Vaccines
WARNINGSInfectionVaccination
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
NURSING MOTHERS
PEDIATRIC USE
ADVERSE REACTIONS
GERIATRIC USE
PREDNISONE ADVERSE REACTIONS
(listed alphabetically, under each subsection)Allergic Reactions
Cardiovascular System
WARNINGSCardio-Renal
Dermatologic
PRECAUTIONSGeneral Precautions
Endocrine
WARNINGSEndocrineWARNINGSEndocrine
Fluid and Electrolyte Disturbances
Gastrointestinal
Hematologic
Metabolic
Musculoskeletal
PRECAUTIONSMusculoskeletal
Neurological/Psychiatric
Ophthalmic
PRECAUTIONSOphthalmic
Other
WARNINGSInfection
DOSAGE & ADMINISTRATION
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT.
Multiple Sclerosis
Alternate Day Therapy
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PrednisonePrednisone TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!