Pravastatin Sodium
Pravastatin Sodium Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- PRAVASTATIN SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PRAVASTATIN SODIUM INDICATIONS AND USAGE
- PRAVASTATIN SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PRAVASTATIN SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- PRAVASTATIN SODIUM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- REFERENCES
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Rx Only
PRAVASTATIN SODIUM DESCRIPTION
Pravastatin sodium tablets are one of a class of lipid-lowering compounds, the HMG-CoA reductase inhibitors, which reduce cholesterol biosynthesis. These agents are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme catalyzing the early rate-limiting step in cholesterol biosynthesis, conversion of HMG- CoA to mevalonate.
Pravastatin sodium is designated chemically as 1-Naphthalene-heptanoic acid, 1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, monosodium salt, [1S-[1α(βS*,δS*),2α,6α,8β(R*),8aα]]-. Structural formula:
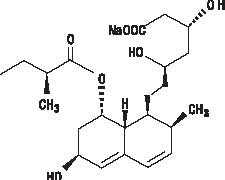
C23 H35 NaO7 MW 446.52
Pravastatin sodium is an odorless, white to off-white, fine or crystalline powder. It is a relatively polar hydrophilic compound with a partition coefficient (octanol/water) of 0.59 at a pH of 7.0. It is soluble in methanol and water (>300 mg/mL), slightly soluble in isopropanol, and practically insoluble in acetone, acetonitrile, chloroform, and ether.
Pravastatin sodium is available for oral administration as 10 mg, 20 mg, 40 mg, and 80 mg tablets. Inactive ingredients include: colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, magnesium stearate, mannitol, meglumine, microcrystalline cellulose and starch. The 10 mg, 20 mg and 80 mg tablets also contain D&C Yellow No 10 Aluminium Lake and the 40 mg tablet also contains D&C Yellow No 10 Aluminium Lake and FD&C Blue No. 1-Aluminium Lake.
CLINICAL PHARMACOLOGY
Cholesterol and triglycerides in the bloodstream circulate as part of lipoprotein complexes. These complexes can be separated by density ultracentrifugation into high (HDL), intermediate (IDL), low (LDL), and very low (VLDL) density lipoprotein fractions. Triglycerides (TG) and cholesterol synthesized in the liver are incorporated into very low density lipoproteins (VLDLs) and released into the plasma for delivery to peripheral tissues. In a series of subsequent steps, VLDLs are transformed into intermediate density lipoproteins (IDLs), and cholesterol-rich low density lipoproteins (LDLs). High density lipoproteins (HDLs), containing apolipoprotein A, are hypothesized to participate in the reverse transport of cholesterol from tissues back to the liver.
Pravastatin sodium produces its lipid-lowering effect in two ways. First, as a consequence of its reversible inhibition of HMG-CoA reductase activity, it effects modest reductions in intracellular pools of cholesterol. This results in an increase in the number of LDL-receptors on cell surfaces and enhanced receptor-mediated catabolism and clearance of circulating LDL. Second, pravastatin inhibits LDL production by inhibiting hepatic synthesis of VLDL, the LDL precursor.
Clinical and pathologic studies have shown that elevated levels of total cholesterol (Total-C), low density lipoprotein cholesterol (LDL-C), and apolipoprotein B (Apo B - a membrane transport complex for LDL) promote human atherosclerosis. Similarly, decreased levels of HDL-cholesterol (HDL-C) and its transport complex, apolipoprotein A, are associated with the development of atherosclerosis. Epidemiologic investigations have established that cardiovascular morbidity and mortality vary directly with the level of Total-C and LDL-C and inversely with the level of HDL-C. Like LDL, cholesterol-enriched triglyceride-rich lipoproteins, including VLDL, IDL, and remnants, can also promote atherosclerosis. Elevated plasma TG are frequently found in a triad with low HDL-C levels and small LDL particles, as well as in association with non-lipid metabolic risk factors for coronary heart disease. As such, total plasma TG has not consistently been shown to be an independent risk factor for CHD. Furthermore, the independent effect of raising HDL or lowering TG on the risk of coronary and cardiovascular morbidity and mortality has not been determined. In both normal volunteers and patients with hypercholesterolemia, treatment with pravastatin sodium tablets reduced Total-C, LDL-C, and apolipoprotein B. Pravastatin sodium also reduced VLDL-C and TG and produced increases in HDL-C and apolipoprotein A. The effects of pravastatin on Lp (a), fibrinogen, and certain other independent biochemical risk markers for coronary heart disease are unknown. Although pravastatin is relatively more hydrophilic than other HMG-CoA reductase inhibitors, the effect of relative hydrophilicity, if any, on either efficacy or safety has not been established.
In one primary (West of Scotland Coronary Prevention Study – WOS)1 prevention study, pravastatin sodium has been shown to reduce cardiovascular morbidity and mortality across a wide range of cholesterol levels (see Clinical Studies ).
Pharmacokinetics/Metabolism
Pravastatin sodium is administered orally in the active form. In clinical pharmacology studies in man, pravastatin is rapidly absorbed, with peak plasma levels of parent compound attained 1 to 1.5 hours following ingestion. Based on urinary recovery of radiolabeled drug, the average oral absorption of pravastatin is 34% and absolute bioavailability is 17%. While the presence of food in the gastrointestinal tract reduces systemic bioavailability, the lipid-lowering effects of the drug are similar whether taken with, or 1 hour prior, to meals.
Pravastatin undergoes extensive first-pass extraction in the liver (extraction ratio 0.66), which is its primary site of action, and the primary site of cholesterol synthesis and of LDL-C clearance. In vitro studies demonstrated that pravastatin is transported into hepatocytes with substantially less uptake into other cells. In view of pravastatin’s apparently extensive first-pass hepatic metabolism, plasma levels may not necessarily correlate perfectly with lipid-lowering efficacy. Pravastatin sodium plasma concentrations [including: area under the concentration-time curve (AUC), peak (Cmax), and steady-state minimum (Cmin)] are directly proportional to administered dose. Systemic bioavailability of pravastatin administered following a bedtime dose was decreased 60% compared to that following an AM dose. Despite this decrease in systemic bioavailability, the efficacy of pravastatin administered once daily in the evening, although not statistically significant, was marginally more effective than that after a morning dose. This finding of lower systemic bioavailability suggests greater hepatic extraction of the drug following the evening dose. Steady-state AUCs, Cmax and Cmin plasma concentrations showed no evidence of pravastatin accumulation following once or twice daily administration of pravastatin sodium tablets. Approximately 50% of the circulating drug is bound to plasma proteins. Following single dose administration of 14C-pravastatin, the elimination half-life (t½) for total radioactivity (pravastatin plus metabolites) in humans is 77 hours.
Pravastatin, like other HMG-CoA reductase inhibitors, has variable bioavailability. The coefficient of variation (CV), based on between-subject variability, was 50% to 60% for AUC. Pravastatin 20 mg was administered under fasting conditions in adults. The geometric means of Cmax and AUC ranged from 23.3 to 26.3 ng/mL and from 54.7 to 62.2 ng*hr/mL, respectively.
Approximately 20% of a radiolabeled oral dose is excreted in urine and 70% in the feces. After intravenous administration of radiolabeled pravastatin to normal volunteers, approximately 47% of total body clearance was via renal excretion and 53% by non-renal routes (i.e., biliary excretion and biotransformation). Since there are dual routes of elimination, the potential exists both for compensatory excretion by the alternate route as well as for accumulation of drug and/or metabolites in patients with renal or hepatic insufficiency.
In a study comparing the kinetics of pravastatin in patients with biopsy confirmed cirrhosis (N=7) and normal subjects (N=7), the mean AUC varied 18-fold in cirrhotic patients and 5-fold in healthy subjects. Similarly, the peak pravastatin values varied 47-fold for cirrhotic patients compared to 6-fold for healthy subjects.
Biotransformation pathways elucidated for pravastatin include: (a) isomerization to 6-epipravastatin and the 3α –hydroxyisomer of pravastatin (SQ 31,906), (b) enzymatic ring hydroxylation to SQ 31,945, (c)ω-1 oxidation of the ester side chain, (d) β-oxidation of the carboxy side chain, (e) ring oxidation followed by aromatization, (f) oxidation of a hydroxyl group to a keto group, and (g) conjugation. The major degradation product is the 3α-hydroxy isomeric metabolite, which has one-tenth to one-fortieth the HMG-CoA reductase inhibitory activity of the parent compound.
In a single oral dose study using pravastatin sodium tablet 20 mg, the mean AUC for pravastatin was approximately 27% greater and the mean cumulative urinary excretion (CUE) approximately 19% lower in elderly men (65 to 75 years old) compared with younger men (19 to 31 years old). In a similar study conducted in women, the mean AUC for pravastatin was approximately 46% higher and the mean CUE approximately 18% lower in elderly women (65 to 78 years old) compared with younger women (18 to 38 years old). In both studies, Cmax, Tmax and t½ values were similar in older and younger subjects.
After two weeks of once-daily 20 mg oral pravastatin administration, the geometric means of AUC were 80.7 (CV 44%) and 44.8 (CV 89%) ng*hr/mL for children (8-11 years, N=14) and adolescents (12-16 years, N=10), respectively. The corresponding values for Cmax were 42.4 (CV 54%) and 18.6 ng/mL (CV 100%) for children and adolescents, respectively. No conclusion can be made based on these findings due to the small number of samples and large variability.
Clinical Studies
Prevention of Coronary Heart Disease
In the Pravastatin Primary Prevention Study (West of Scotland Coronary Prevention Study –WOS),1 the effect of pravastatin sodium on fatal and nonfatal coronary heart disease (CHD) was assessed in 6595 men 45-64 years of age, without a previous myocardial infarction (MI), and with LDL-C levels between 156-254 mg/dL (4-6.7 mmol/L). In this randomized, double-blind, placebo-controlled study, patients were treated with standard care, including dietary advice, and either pravastatin sodium tablet 40 mg daily (N=3302) or placebo (N=3293) and followed for a median duration of 4.8 years. Median (25th, 75th percentile) percent changes from baseline after 6 months of pravastatin treatment in Total C, LDL-C, TG, and HDL were -20.3 (-26.9, -11.7), -27.7 (-36.0, -16.9), -9.1 (-27.6, 12.5), and 6.7 (-2.1, 15.6), respectively.
Pravastatin sodium significantly reduced the rate of first coronary events (either coronary heart disease [CHD] death or nonfatal MI) by 31% [248 events in the placebo group (CHD death=44, nonfatal MI=204) vs 174 events in the pravastatin sodium group (CHD death=31, nonfatal MI=143), p=0.0001 (see figure below)]. The risk reduction with pravastatin sodium was similar and significant throughout the entire range of baseline LDL cholesterol levels. This reduction was also similar and significant across the age range studied with a 40% risk reduction for patients younger than 55 years and a 27% risk reduction for patients 55 years and older. The Pravastatin Primary Prevention Study included only men and therefore it is not clear to what extent these data can be extrapolated to a similar population of female patients.
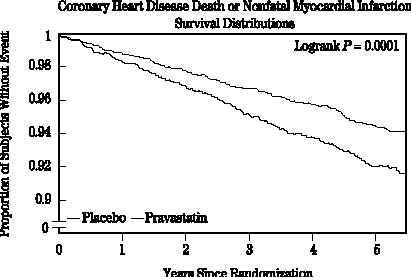
Pravastatin sodium also significantly decreased the risk for undergoing myocardial revascularization procedures (coronary artery bypass graft [CABG] surgery or percutaneous transluminal coronary angioplasty [PTCA]) by 37% (80 vs 51 patients, p=0.009) and coronary angiography by 31% (128 vs 90, p=0.007). Cardiovascular deaths were decreased by 32% (73 vs 50, p=0.03) and there was no increase in death from non-cardiovascular causes.
Secondary Prevention of Cardiovascular Events
In the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC I)4 study, the effect of pravastatin therapy on coronary atherosclerosis was assessed by coronary angiography in patients with coronary disease and moderate hypercholesterolemia (baseline LDL-C range=130-190 mg/dL). In this double-blind, multicenter, controlled clinical trial angiograms were evaluated at baseline and at three years in 264 patients. Although the difference between pravastatin and placebo for the primary endpoint (per-patient change in mean coronary artery diameter) and one of two secondary endpoints (change in percent lumen diameter stenosis) did not reach statistical significance, for the secondary endpoint of change in minimum lumen diameter, statistically significant slowing of disease was seen in the pravastatin treatment group (p=0.02).
In the Regression Growth Evaluation Statin Study (REGRESS)5, the effect of pravastatin on coronary atherosclerosis was assessed by coronary angiography in 885 patients with angina pectoris, angiographically documented coronary artery disease and hypercholesterolemia (baseline total cholesterol range=160-310 mg/dL). In this double-blind, multicenter, controlled clinical trial, angiograms were evaluated at baseline and at two years in 653 patients (323 treated with pravastatin). Progression of coronary atherosclerosis was significantly slowed in the pravastatin group as assessed by changes in mean segment diameter (p=0.037) and minimum obstruction diameter (p=0.001).
Analysis of pooled events from PLAC I, the Pravastatin, Lipids and Atherosclerosis in the Carotids Study (PLAC II)6, REGRESS, and the Kuopio Atherosclerosis Prevention Study (KAPS)7 (combined N=1891) showed that treatment with pravastatin was associated with a statistically significant reduction in the composite event rate of fatal and nonfatal myocardial infarction (46 events or 6.4% for placebo versus 21 events or 2.4% for pravastatin, p=0.001). The predominant effect of pravastatin was to reduce the rate of nonfatal myocardial infarction.
Primary Hypercholesterolemia (Fredrickson Type IIa and IIb)
Pravastatin sodium is highly effective in reducing Total-C, LDL-C and triglycerides (TG) in patients with heterozygous familial, presumed familial combined and non-familial (non-FH) forms of primary hypercholesterolemia, and mixed dyslipidemia. A therapeutic response is seen within 1 week, and the maximum response usually is achieved within 4 weeks. This response is maintained during extended periods of therapy. In addition, pravastatin sodium is effective in reducing the risk of acute coronary events in hypercholesterolemic patients with and without previous myocardial infarction.
A single daily dose is as effective as the same total daily dose given twice a day. In multicenter, double-blind, placebo-controlled studies of patients with primary hypercholesterolemia, treatment with pravastatin in daily doses ranging from 10 mg to 40 mg consistently and significantly decreased Total-C, LDL-C, TG, and Total-C/HDL-C and LDL-C/HDL-C ratios (see Table 1).
In a pooled analysis of two multicenter, double-blind, placebo-controlled studies of patients with primary hypercholesterolemia, treatment with pravastatin at a daily dose of 80 mg (N=277) significantly decreased Total-C, LDL-C, and TG. The 25th and 75th percentile changes from baseline in LDL-C for pravastatin 80 mg were -43% and -30%. The efficacy results of the individual studies were consistent with the pooled data (see Table 1).
Treatment with pravastatin sodium modestly decreased VLDL-C and pravastatin sodium across all doses produced variable increases in HDL-C (see Table 1).
| Dose | Total-C | LDL-C | HDL-C | TG |
Mean Percent Changes From Baseline After 8 Weeks |
||||
| Placebo (N=36) | -3% | -4% | +1% | -4% |
| 10 mg (N=18) | -16% | -22% | +7% | -15% |
| 20 mg (N=19) | -24% | -32% | +2% | -11% |
| 40 mg (N=18) | -25% | -34% | +12% | -24% |
Mean Percent Changes From Baseline After 6 Weeks |
||||
| Placebo (N=162) | 0% | -1% | -1% | +1% |
| 80 mg (N=277) | -27% | -37% | +3% | -19% |
In another clinical trial, patients treated with pravastatin in combination with cholestyramine (70% of patients were taking cholestyramine 20 or 24 g per day) had reductions equal to or greater than 50% in LDL-C. Furthermore, pravastatin attenuated cholestyramine-induced increases in TG levels (which are themselves of uncertain clinical significance).
Hypertriglyceridemia (Fredrickson Type IV)
The response to pravastatin in patients with Type IV hyperlipidemia (baseline TG >200 mg/dL and LDL-C <160 mg/dL) was evaluated in a subset of 429 patients. For pravastatin-treated subjects, the median (min, max) baseline triglyceride level was 246.0 (200.5, 349.5) mg/dL (see Table 2).
| Pravastatin 40 mg (N=429) | Placebo (N=430) | |
| Triglycerides | -21.1 (-34.8, 1.3) | -6.3 (-23.1, 18.3) |
| Total-C | -22.1 (-27.1, -14.8) | 0.2 (-6.9, 6.8) |
| LDL-C | -31.7 (-39.6, -21.5) | 0.7 (-9.0, 10.0) |
| HDL-C | 7.4 (-1.2, 17.7) | 2.8 (-5.7, 11.7) |
| Non-HDL-C | -27.2 (-34.0, -18.5) | -0.8 (-8.2, 7.0) |
Dysbetalipoproteinemia (Fredrickson Type III)
The response to pravastatin in two double-blind crossover studies of 46 patients with genotype E2/E2 and Fredrickson Type III dysbetalipoproteinemia is shown in Table 3.
| Median (min, max) at Baseline (mg/dL) | Median % Change (min, max) Pravastatin 40 mg (N=20) | |
| Study 1 | ||
| Total-C | 386.5 (245.0, 672.0) | -32.7 (-58.5, 4.6) |
| Triglycerides | 443.0 (275.0, 1299.0) | -23.7 (-68.5, 44.7) |
| VLDL-C* | 206.5 (110.0, 379.0) | -43.8 (-73.1, -14.3) |
| LDL-C* | 117.5 (80.0, 170.0) | -40.8 (-63.7, 4.6) |
| HDL-C | 30.0 (18.0, 88.0) | 6.4 (-45.0, 105.6) |
| Non-HDL-C | 344.5 (215.0, 646.0) | -36.7 (-66.3, 5.8) |
| *N=14 | ||
| Median (min, max) at Baseline (mg/dL) | Median % Change (min, max) Pravastatin 40 mg (N=26) | |
| Study 2 | ||
| Total-C | 340.3 (230.1, 448.6) | -31.4 (-54.5, -13.0) |
| Triglycerides | 343.2 (212.6, 845.9) | -11.9 (-56.5, 44.8) |
| VLDL-C | 145.0 (71.5, 309.4) | -35.7 (-74.7, 19.1) |
| LDL-C | 128.6 (63.8, 177.9) | -30.3 (-52.2, 13.5) |
| HDL-C | 38.7 (27.1, 58.0) | 5.0 (-17.7, 66.7) |
| Non-HDL-C | 295.8 (195.3, 421.5) | -35.5 (-81.0, -13.5) |
Pediatric Clinical Study
A double-blind placebo-controlled study in 214 patients (100 boys and 114 girls) with heterozygous familial hypercholesterolemia (HeFH), aged 8-18 years was conducted for two (2) years. The children (aged 8-13 years) were randomized to placebo (N=63) or 20 mg of pravastatin daily (N=65) and the adolescents (aged 14-18 years) were randomized to placebo (N=45) or 40 mg of pravastatin daily (N=41). Inclusion in the study required LDL-C level >95th percentile for age and sex and one parent with either a clinical or molecular diagnosis of familial hypercholesterolemia. The mean baseline LDL-C value was 239 mg/dL and 237 mg/dL in the pravastatin (range: 151-405 mg/dL) and placebo (range: 154-375 mg/dL) groups, respectively.
Pravastatin significantly decreased plasma levels of LDL-C, Total-C, and apolipoprotein B in both children and adolescents (see Table 4). The effect of pravastatin treatment in the two age groups was similar.
| 95% CI of the | |||||
|
Pravastatin 20 mg (Aged 8-13 years) N=65 |
Pravastatin 40 mg (Aged 14-18 years) N=41 |
Combined Pravastatin (Aged 8-18 years) N=106 |
Combined Placebo (Aged 8-18 years) N=108 |
Difference Between Combined Pravastatin and Placebo |
|
| LDL-C | -26.04 |
-21.07 |
-24.07 |
-1.52 | (-26.74, -18.86) |
| TC | -20.75 |
-13.08 |
-17.72 |
-0.65 | (-20.40, -13.83) |
| HDL-C | 1.04 | 13.71 | 5.97 | 3.13 | (-1.71, 7.43) |
| TG | -9.58 | -0.30 | -5.88 | -3.27 | (-13.95, 10.01) |
| ApoB | -23.16 |
-18.08 |
-21.11 |
-0.97 | (-24.29, -16.18) |
| (N) | (61) | (39) | (100) | (106) | |
The mean achieved LDL-C was 186 mg/dL (range: 67-363 mg/dL) in the pravastatin group compared to 236 mg/dL (range: 105-438 mg/dL) in the placebo group.
The safety and efficacy of pravastatin doses above 40 mg daily have not been studied in children. The long-term efficacy of pravastatin therapy in childhood to reduce morbidity and mortality in adulthood has not been established.
PRAVASTATIN SODIUM INDICATIONS AND USAGE
Therapy with pravastatin sodium tablets should be considered in those individuals at increased risk for atherosclerosis-related clinical events as a function of cholesterol level, the presence or absence of coronary heart disease, and other risk factors.
Primary Prevention of Coronary Events
In hypercholesterolemic patients without clinically evident coronary heart disease, pravastatin sodium is indicated to:
- Reduce the risk of myocardial infarction
- Reduce the risk of undergoing myocardial revascularization procedures
- Reduce the risk of cardiovascular mortality with no increase in death from non-cardiovascular causes.
Hyperlipidemia
Pravastatin sodium tablets are indicated as an adjunct to diet to reduce elevated Total-C, LDL-C, Apo B, and TG levels and to increase HDL-C in patients with primary hypercholesterolemia and mixed dyslipidemia (Fredrickson Type IIa and IIb).8
Pravastatin sodium tablets are indicated as adjunctive therapy to diet for the treatment of patients with elevated serum triglyceride levels (Fredrickson Type IV).
Pravastatin sodium tablets are indicated for the treatment of patients with primary dysbetalipoproteinemia (Fredrickson Type III) who do not respond adequately to diet.
Pravastatin sodium tablets are indicated as an adjunct to diet and life-style modification for treatment of HeFH in children and adolescent patients ages 8 years and older if after an adequate trial of diet the following findings are present.
- LDL-C remains ≥190 mg/dL or
- LDL-C remains ≥160 mg/dL and;
- there is a positive family history of premature cardiovascular disease or
- two or more other CVD risk factors are present in the patient.
Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol when the response to diet and other nonpharmacological measures alone has been inadequate (see NCEP Guidelines below).
Prior to initiating therapy with pravastatin, secondary causes for hypercholesterolemia (e.g., poorly controlled diabetes mellitus, hypothyroidism, nephrotic syndrome, dysproteinemias, obstructive liver disease, other drug therapy, alcoholism) should be excluded, and a lipid profile performed to measure Total-C, HDL-C, and TG. For patients with triglycerides (TG) <400 mg/dL (<4.5 mmol/L), LDL-C can be estimated using the following equation:
LDL-C = Total-C - HDL-C - 1/5 TG
For TG levels >400 mg/dL (>4.5 mmol/L), this equation is less accurate and LDL-C concentrations should be determined by ultracentrifugation. In many hypertriglyceridemic patients, LDL-C may be low or normal despite elevated Total-C. In such cases, HMG-CoA reductase inhibitors are not indicated.
Lipid determinations should be performed at intervals of no less than four weeks and dosage adjusted according to the patient’s response to therapy.
The National Cholesterol Education Program’s Treatment Guidelines are summarized below:
| Risk Category | LDL Goal (mg/dL) |
LDL Level at Which to Initiate Therapeutic Lifestyle Changes (mg/dL) |
LDL Levels at Which to Consider Drug Therapy (mg/dL) |
|
CHD |
<100 | ≥100 |
≥130 (100-129: drug optional) |
|
2+ Risk factors (10-year risk ≤20%) |
<130 | ≥130 |
10-year risk 10%-20%: ≥130 |
| 10-year risk <10%: ≥160 | |||
0-1 Risk factor |
<160 | ≥160 |
≥190 (160-189: LDL-lowering drug optional) |
After the LDL-C goal has been achieved, if the TG is still ≥200 mg/dL, non-HDL-C (Total-C minus HDL-C) becomes a secondary target of therapy. Non-HDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category.
At the time of hospitalization for an acute coronary event, consideration can be given to initiating drug therapy at discharge if the LDL-C is ≥130 mg/dL (see NCEP Treatment Guidelines, above).
Since the goal of treatment is to lower LDL-C, the NCEP recommends that LDL-C levels be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the Total-C be used to monitor therapy.
As with other lipid-lowering therapy, pravastatin sodium tablets are not indicated when hypercholesterolemia is due to hyperalphalipoproteinemia (elevated HDL-C).
The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature cardiovascular disease is summarized below:
| Category | Total-C (mg/dL) | LDL-C (mg/dL) |
| Acceptable | <170 | <110 |
| Borderline | 170-199 | 110-129 |
| High | ≥200 | ≥130 |
PRAVASTATIN SODIUM CONTRAINDICATIONS
Hypersensitivity to any component of this medication.
Active liver disease or unexplained, persistent elevations of serum transaminases (see WARNINGS ).
Pregnancy and Lactation.
Atherosclerosis is a chronic process and discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. Cholesterol and other products of cholesterol biosynthesis are essential components for fetal development (including synthesis of steroids and cell membranes). Since HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, they are contraindicated during pregnancy and in nursing mothers. Pravastatin should be administered to women of childbearing age only when such patients are highly unlikely to conceive and have been informed of the potential hazards. If the patient becomes pregnant while taking this class of drug, therapy should be discontinued immediately and the patient apprised of the potential hazard to the fetus (see PRECAUTIONS: Pregnancy ).
WARNINGS
Liver Enzymes
HMG-CoA reductase inhibitors, like some other lipid-lowering therapies, have been associated with biochemical abnormalities of liver function. In placebo-controlled trials (see CLINICAL PHARMACOLOGY: Clinical Studies ), subjects were exposed to pravastatin or placebo. In an analysis of serum transaminase values (ALT, AST), incidences of marked abnormalities were compared between the pravastatin and placebo treatment groups; a marked abnormality was defined as a post-treatment test value greater than three times the upper limit of normal for subjects with pretreatment values less than or equal to the upper limit of normal, or four times the pretreatment value for subjects with pretreatment values greater than the upper limit of normal but less than 1.5 times the upper limit of normal. Marked abnormalities of ALT or AST occurred with similar low frequency (≤1.2%) in both treatment groups. Overall, clinical trial experience showed that liver function test abnormalities observed during pravastatin therapy were usually asymptomatic, not associated with cholestasis, and did not appear to be related to treatment duration. In a 320-patient placebo-controlled clinical trial, subjects with chronic (>6 months) stable liver disease, due primarily to hepatitis C or non-alcoholic fatty liver disease, were treated with 80 mg pravastatin or placebo for up to 9 months. The primary safety endpoint was the proportion of subjects with at least one ALT ≥2 times the upper limit of normal for those with normal ALT (≤ the upper limit of normal) at baseline or a doubling of the baseline ALT for those with elevated ALT (> the upper limit of normal) at baseline. By Week 36, 12 out of 160 (7.5%) subjects treated with pravastatin met the prespecified safety ALT endpoint compared to 20 out of 160 (12.5%) subjects receiving placebo. Conclusions regarding liver safety are limited since the study was not large enough to establish similarity between groups (with 95% confidence) in the rates of ALT elevation.
It is recommended that liver function tests be performed prior to the initiation of therapy and when clinically indicated.
Active liver disease or unexplained persistent transaminase elevations are contraindications to the use of pravastatin (see CONTRAINDICATIONS ). Caution should be exercised when pravastatin is administered to patients who have a recent (< 6 months) history of liver disease, have signs that may suggest liver disease (e.g., unexplained aminotransferase elevations, jaundice), or are heavy users of alcohol (see CLINICAL PHARMACOLOGY: Pharmacokinetics/Metabolism ). Such patients should be closely monitored, started at the lower end of the recommended dosing range (see DOSAGE AND ADMINISTRATION: Adult Patients ), and titrated to the desired therapeutic effect.
Patients who develop increased transaminase levels or signs and symptoms of active liver disease while taking pravastatin should be evaluated with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality(ies) return to normal. Should an increase in AST or ALT of three times the upper limit of normal or greater persist, withdrawal of pravastatin therapy is recommended.
Skeletal Muscle
Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with pravastatin and other drugs in this class. Uncomplicated myalgia has also been reported in pravastatin-treated patients (see ADVERSE REACTIONS ). Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in creatine phosphokinase (CPK) values to greater than 10 times the upper limit of normal, was rare (<0.1%) in pravastatin clinical trials. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevation of CPK. Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. Pravastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Pravastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
The risk of myopathy during treatment with another HMG-CoA reductase inhibitor is increased with concurrent therapy with either erythromycin, cyclosporine, niacin, or fibrates. However, neither myopathy nor significant increases in CPK levels have been observed in three reports involving a total of 100 post-transplant patients (24 renal and 76 cardiac) treated for up to two years concurrently with pravastatin 10-40 mg and cyclosporine. Some of these patients also received other concomitant immunosuppressive therapies. Further, in clinical trials involving small numbers of patients who were treated concurrently with pravastatin and niacin, there were no reports of myopathy. Also, myopathy was not reported in a trial of combination pravastatin (40 mg/day) and gemfibrozil (1200 mg/day), although 4 of 75 patients on the combination showed marked CPK elevations versus one of 73 patients receiving placebo. There was a trend toward more frequent CPK elevations and patient withdrawals due to musculoskeletal symptoms in the group receiving combined treatment as compared with the groups receiving placebo, gemfibrozil, or pravastatin monotherapy (see PRECAUTIONS: Drug interactions ). The use of fibrates alone may occasionally be associated with myopathy. The combined use of pravastatin and fibrates should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination.
PRECAUTIONS
General
Pravastatin sodium may elevate creatine phosphokinase and transaminase levels (see ADVERSE REACTIONS ). This should be considered in the differential diagnosis of chest pain in a patient on therapy with pravastatin.
Homozygous Familial Hypercholesterolemia.
Pravastatin has not been evaluated in patients with rare homozygous familial hypercholesterolemia. In this group of patients, it has been reported that HMG-CoA reductase inhibitors are less effective because the patients lack functional LDL receptors.
Renal Insufficiency.
A single 20 mg oral dose of pravastatin was administered to 24 patients with varying degrees of renal impairment (as determined by creatinine clearance). No effect was observed on the pharmacokinetics of pravastatin or its 3α-hydroxy isomeric metabolite (SQ 31,906). A small increase was seen in mean AUC values and half-life (t½) for the inactive enzymatic ring hydroxylation metabolite (SQ 31,945). Given this small sample size, the dosage administered, and the degree of individual variability, patients with renal impairment who are receiving pravastatin should be closely monitored.
Information for patients
Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever (see WARNINGS: Skeletal Muscle ).
Drug interactions
Immunosuppressive Drugs, Gemfibrozil, Niacin (Nicotinic Acid), Erythromycin : See WARNINGS: Skeletal Muscle .
Cytochrome P450 3A4 Inhibitors : In vitro and in vivo data indicate that pravastatin is not metabolized by cytochrome P450 3A4 to a clinically significant extent. This has been shown in studies with known cytochrome P450 3A4 inhibitors (see Diltiazem and Itraconazole below). Other examples of cytochrome P450 3A4 inhibitors include ketoconazole, mibefradil, and erythromycin.
Diltiazem : Steady-state levels of diltiazem (a known, weak inhibitor of P450 3A4) had no effect on the pharmacokinetics of pravastatin. In this study, the AUC and Cmax of another HMG-CoA reductase inhibitor which is known to be metabolized by cytochrome P450 3A4 increased by factors of 3.6 and 4.3, respectively.
Itraconazole : The mean AUC and Cmax for pravastatin were increased by factors of 1.7 and 2.5, respectively, when given with itraconazole (a potent P450 3A4 inhibitor which also inhibits p-glycoprotein transport) as compared to placebo. The mean t½ was not affected by itraconazole, suggesting that the relatively small increases in Cmax and AUC were due solely to increased bioavailability rather than a decrease in clearance, consistent with inhibition of p-glycoprotein transport by itraconazole. This drug transport system is thought to affect bioavailability and excretion of HMG-CoA reductase inhibitors, including pravastatin. The AUC and Cmax of another HMG-CoA reductase inhibitor which is known to be metabolized by cytochrome P450 3A4 increased by factors of 19 and 17, respectively, when given with itraconazole.
Antipyrine : Since concomitant administration of pravastatin had no effect on the clearance of antipyrine, interactions with other drugs metabolized via the same hepatic cytochrome isozymes are not expected.
Cholestyramine/Colestipol: Concomitant administration resulted in an approximately 40 to 50% decrease in the mean AUC of pravastatin. However, when pravastatin was administered 1 hour before or 4 hours after cholestyramine or 1 hour before colestipol and a standard meal, there was no clinically significant decrease in bioavailability or therapeutic effect. (See DOSAGE AND ADMINISTRATION: Concomitant Therapy .)
Warfarin : Concomitant administration of 40 mg pravastatin had no clinically significant effect on prothrombin time when administered in a study to normal elderly subjects who were stabilized on warfarin.
Cimetidine : The AUC0-12hr for pravastatin when given with cimetidine was not significantly different from the AUC for pravastatin when given alone. A significant difference was observed between the AUC’s for pravastatin when given with cimetidine compared to when administered with antacid.
Digoxin: In a crossover trial involving 18 healthy male subjects given 20 mg pravastatin and 0.2 mg digoxin concurrently for 9 days, the bioavailability parameters of digoxin were not affected. The AUC of pravastatin tended to increase, but the overall bioavailability of pravastatin plus its metabolites SQ 31,906 and SQ 31,945 was not altered.
Cyclosporine : Some investigators have measured cyclosporine levels in patients on pravastatin (up to 20 mg), and to date, these results indicate no clinically meaningful elevations in cyclosporine levels. In one single-dose study, pravastatin levels were found to be increased in cardiac transplant patients receiving cyclosporine.
Gemfibrozil: In a crossover study in 20 healthy male volunteers given concomitant single doses of pravastatin and gemfibrozil, there was a significant decrease in urinary excretion and protein binding of pravastatin. In addition, there was a significant increase in AUC, Cmax, and Tmax for the pravastatin metabolite SQ 31,906. Combination therapy with pravastatin and gemfibrozil is generally not recommended. (See WARNINGS: Skeletal Muscle .)
In interaction studies with aspirin, antacids (1 hour prior to pravastatin) cimetidine, nicotinic acid, or probucol, no statistically significant differences in bioavailability were seen when pravastatin sodium was administered.
Endocrine Function
HMG-CoA reductase inhibitors interfere with cholesterol synthesis and lower circulating cholesterol levels and, as such, might theoretically blunt adrenal or gonadal steroid hormone production. Results of clinical trials with pravastatin in males and post-menopausal females were inconsistent with regard to possible effects of the drug on basal steroid hormone levels. In a study of 21 males, the mean testosterone response to human chorionic gonadotropin was significantly reduced (p<0.004) after 16 weeks of treatment with 40 mg of pravastatin. However, the percentage of patients showing a ≥50% rise in plasma testosterone after human chorionic gonadotropin stimulation did not change significantly after therapy in these patients. The effects of HMG-CoA reductase inhibitors on spermatogenesis and fertility have not been studied in adequate numbers of patients. The effects, if any, of pravastatin on the pituitary-gonadal axis in pre-menopausal females are unknown. Patients treated with pravastatin who display clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should also be exercised if an HMG-CoA reductase inhibitor or other agent used to lower cholesterol levels is administered to patients also receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may diminish the levels or activity of steroid hormones.
In a placebo-controlled study of 214 pediatric patients with HeFH, of which 106 were treated with pravastatin (20 mg in the children aged 8-13 years and 40 mg in the adolescents aged 14-18 years) for two years, there were no detectable differences seen in any of the endocrine parameters [ACTH, cortisol, DHEAS, FSH, LH, TSH, estradiol (girls) or testosterone (boys)] relative to placebo. There were no detectable differences seen in height and weight changes, testicular volume changes, or Tanner score relative to placebo.
CNS Toxicity
CNS vascular lesions, characterized by perivascular hemorrhage and edema and mononuclear cell infiltration of perivascular spaces, were seen in dogs treated with pravastatin at a dose of 25 mg/kg/day. These effects in dogs were observed at approximately 59 times the human dose of 80 mg/day, based on AUC. Similar CNS vascular lesions have been observed with several other drugs in this class.
A chemically similar drug in this class produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion starting at 60 mg/kg/day, a dose that produced mean plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose (as measured by total enzyme inhibitory activity). This same drug also produced vestibulocochlear Wallerian-like degeneration and retinal ganglion cell chromatolysis in dogs treated for 14 weeks at 180 mg/kg/day, a dose which resulted in a mean plasma drug level similar to that seen with the 60 mg/kg/day dose.
Carcinogenesis, mutagenesis, impairment of fertility
In a 2-year study in rats fed pravastatin at doses of 10, 30, or 100 mg/kg body weight, there was an increased incidence of hepatocellular carcinomas in males at the highest dose (p<0.01). These effects in rats were observed at approximately 12 times the human dose (HD) of 80 mg, based on body surface area mg/m2 and at approximately 4 times the human dose, based on AUC.
In a 2-year study in mice fed pravastatin at doses of 250 and 500 mg/kg/day, there was an increased incidence of hepatocellular carcinomas in males and females at both 250 and 500 mg/kg/day (p<0.0001). At these doses, lung adenomas in females were increased (p=0.013). These effects in mice were observed at approximately 15 times (250 mg/kg/day) and 23 times (500 mg/kg/day) the human dose of 80 mg, based on AUC. In another 2-year study in mice with doses up to 100 mg/kg/day (producing drug exposures approximately 2 times the human dose of 80 mg, based on AUC), there were no drug-induced tumors.
No evidence of mutagenicity was observed in vitro, with or without rat-liver metabolic activation, in the following studies: microbial mutagen tests, using mutant strains of Salmonella typhimurium or Escherichia coli; a forward mutation assay in L5178Y TK +/- mouse lymphoma cells; a chromosomal aberration test in hamster cells; and a gene conversion assay using Saccharomyces cerevisiae. In addition, there was no evidence of mutagenicity in either a dominant lethal test in mice or a micronucleus test in mice.
In a study in rats, with daily doses up to 500 mg/kg, pravastatin did not produce any adverse effects on fertility or general reproductive performance. However, in a study with another HMG-CoA reductase inhibitor, there was decreased fertility in male rats treated for 34 weeks at 25 mg/kg body weight, although this effect was not observed in a subsequent fertility study when this same dose was administered for 11 weeks (the entire cycle of spermatogenesis, including epididymal maturation). In rats treated with this same reductase inhibitor at 180 mg/kg/day, seminiferous tubule degeneration (necrosis and loss of spermatogenic epithelium) was observed. Although not seen with pravastatin, two similar drugs in this class caused drug-related testicular atrophy, decreased spermatogenesis, spermatocytic degeneration, and giant cell formation in dogs. The clinical significance of these findings is unclear.
Pregnancy
Pregnancy Category X.
See
CONTRAINDICATIONS
.
Safety in pregnant women has not been established. Pravastatin was not teratogenic in rats at doses up to 1000 mg/kg daily or in rabbits at doses of up to 50 mg/kg daily. These doses resulted in 10X (rabbit) or 120X (rat) the human exposure based on surface area (mg/meter2). Rare reports of congenital anomalies have been received following intrauterine exposure to other HMG-CoA reductase inhibitors. In a review9 of approximately 100 prospectively followed pregnancies in women exposed to simvastatin or lovastatin, the incidences of congenital anomalies, spontaneous abortions and fetal deaths/stillbirths did not exceed what would be expected in the general population. The number of cases is adequate only to exclude a three-to-four-fold increase in congenital anomalies over the background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified. As safety in pregnant women has not been established and there is no apparent benefit to therapy with pravastatin during pregnancy (see CONTRAINDICATIONS ), treatment should be immediately discontinued as soon as pregnancy is recognized. Pravastatin sodium should be administered to women of child-bearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards.
Nursing mothers
A small amount of pravastatin is excreted in human breast milk. Because of the potential for serious adverse reactions in nursing infants, women taking pravastatin sodium should not nurse (see CONTRAINDICATIONS ).
Pediatric use
The safety and effectiveness of pravastatin sodium in children and adolescents from 8-18 years of age have been evaluated in a placebo-controlled study of 2 years duration. Patients treated with pravastatin had an adverse experience profile generally similar to that of patients treated with placebo with influenza and headache commonly reported in both treatment groups. (See ADVERSE REACTIONS: Pediatric Patients .) Doses greater than 40 mg have not been studied in this population. Children and adolescent females of childbearing potential should be counseled on appropriate contraceptive methods while on pravastatin therapy (see CONTRAINDICATIONS and Pregnancy ). For dosing information see DOSAGE AND ADMINISTRATION: Adult Patients and Pediatric Patients .
Double-blind, placebo-controlled pravastatin studies in children less than 8 years of age have not been conducted.
Geriatric use
The beneficial effect of pravastatin in elderly subjects in reducing cardiovascular events and in modifying lipid profiles was similar to that seen in younger subjects. The adverse event profile in the elderly was similar to that in the overall population. Other reported clinical experience has not identified differences in responses to pravastatin between elderly and younger patients.
Mean pravastatin AUCs are slightly (25-50%) higher in elderly subjects than in healthy young subjects, but mean Cmax, Tmax and t1/2 values are similar in both age groups and substantial accumulation of pravastatin would not be expected in the elderly (see CLINICAL PHARMACOLOGY: Pharmacokinetics/Metabolism ).
PRAVASTATIN SODIUM ADVERSE REACTIONS
Pravastatin is generally well tolerated; adverse reactions have usually been mild and transient. In 4-month long placebo-controlled trials, 1.7% of pravastatin-treated patients and 1.2% of placebo-treated patients were discontinued from treatment because of adverse experiences attributed to study drug therapy; this difference was not statistically significant. (See also PRECAUTIONS: Geriatric use ).
Adverse Clinical Events
Short-Term Controlled Trials
All adverse clinical events (regardless of attribution) reported in more than 2% of pravastatin-treated patients in placebo-controlled trials of up to four months duration are identified in Table 6; also shown are the percentages of patients in whom these medical events were believed to be related or possibly related to the drug:
| 10-40 mg in Short-Term Placebo-Controlled Trials | ||||
| All Events | Events Attributed to Study Drug | |||
| Body System/Event |
Pravastatin (N=900) % of patients |
Placebo (N=411) % of patients |
Pravastatin (N=900) % of patients |
Placebo (N=411) % of patients |
| Cardiovascular | ||||
| Cardiac Chest Pain | 4.0 | 3.4 | 0.1 | 0.0 |
| Dermatologic | ||||
| Rash | 4.0* | 1.1 | 1.3 | 0.9 |
| Gastrointestinal | ||||
| Nausea/Vomiting | 7.3 | 7.1 | 2.9 | 3.4 |
| Diarrhea | 6.2 | 5.6 | 2.0 | 1.9 |
| Abdominal Pain | 5.4 | 6.9 | 2.0 | 3.9 |
| Constipation | 4.0 | 7.1 | 2.4 | 5.1 |
| Flatulence | 3.3 | 3.6 | 2.7 | 3.4 |
| Heartburn | 2.9 | 1.9 | 2.0 | 0.7 |
| General | ||||
| Fatigue | 3.8 | 3.4 | 1.9 | 1.0 |
| Chest Pain | 3.7 | 1.9 | 0.3 | 0.2 |
| Influenza | 2.4 |
0.7 | 0.0 | 0.0 |
| Musculoskeletal | ||||
| Localized Pain | 10.0 | 9.0 | 1.4 | 1.5 |
| Myalgia | 2.7 | 1.0 | 0.6 | 0.0 |
| Nervous System | ||||
| Headache | 6.2 | 3.9 | 1.7* | 0.2 |
| Dizziness | 3.3 | 3.2 | 1.0 | 0.5 |
| Renal/Genitourinary | ||||
|
Urinary Abnormality |
2.4 | 2.9 | 0.7 | 1.2 |
| Respiratory | ||||
| Common Cold | 7.0 | 6.3 | 0.0 | 0.0 |
| Rhinitis | 4.0 | 4.1 | 0.1 | 0.0 |
| Cough | 2.6 | 1.7 | 0.1 | 0.0 |
The safety and tolerability of pravastatin sodium at a dose of 80 mg in two controlled trials with a mean exposure of 8.6 months was similar to that of pravastatin sodium at lower doses except that 4 out of 464 patients taking 80 mg of pravastatin had a single elevation of CK >10X ULN compared to 0 out of 115 patients taking 40 mg of pravastatin.
Long-Term Controlled Morbidity and Mortality Trials
Adverse event data were pooled from several double-blind, placebo-controlled trials (West of Scotland Coronary Prevention study [WOS]; Pravastatin Limitation of Atherosclerosis in the Coronary Arteries study [PLAC I]; Pravastatin, Lipids and Atherosclerosis in the Carotids study [PLAC II]; Regression Growth Evaluation Statin Study [REGRESS]; and Kuopio Atherosclerosis Prevention Study [KAPS]) involving a total of 10,764 patients treated with pravastatin 40 mg and 10,719 patients treated with placebo. The safety and tolerability profile in the pravastatin group was comparable to that of the placebo group. Patients were exposed to pravastatin for a mean of 4.0 to 5.1 years in WOS and 1.9 to 2.9 years in PLAC I, PLAC II, KAPS, and REGRESS. In these long-term trials, the most common reasons for discontinuation were mild, non-specific gastrointestinal complaints. Collectively, these seven trials represent 47,613 patient-years of exposure to pravastatin. Events believed to be of probable, possible, or uncertain relationship to study drug, occurring in at least 1% of patients treated with pravastatin in these studies are identified in Table 7.
| 40 mg in Long-Term Placebo-Controlled Trials | ||
| Body System/Event |
Pravastatin (N=10,764) % of patients |
Placebo (N=10,719) % of patients |
| Cardiovascular | ||
| Angina Pectoris | 3.1 | 3.4 |
| Dermatologic | ||
| Rash | 2.1 | 2.2 |
| Gastrointestinal | ||
| Dyspepsia/Heartburn | 3.5 | 3.7 |
| Abdominal Pain | 2.4 | 2.5 |
| Nausea/Vomiting | 1.6 | 1.6 |
| Flatulence | 1.2 | 1.1 |
| Constipation | 1.2 | 1.3 |
| General | ||
| Fatigue | 3.4 | 3.3 |
| Chest Pain | 2.6 | 2.6 |
| Musculoskeletal | ||
| Musculoskeletal Pain (includes arthralgia) | 6.0 | 5.8 |
| Muscle Cramp | 2.0 | 1.8 |
| Myalgia | 1.4 | 1.4 |
| Nervous System | ||
| Dizziness | 2.2 | 2.1 |
| Headache | 1.9 | 1.8 |
| Sleep Disturbance | 1.0 | 0.9 |
| Depression | 1.0 | 1.0 |
| Anxiety/Nervousness | 1.0 | 1.2 |
| Renal/Genitourinary | ||
|
Urinary Abnormality (includes dysuria, frequency, nocturia) |
1.0 | 0.8 |
| Respiratory | ||
| Dyspnea | 1.6 | 1.6 |
| Upper Respiratory Infection | 1.3 | 1.3 |
| Cough | 1.0 | 1.0 |
| Special Senses | ||
| Vision Disturbance (includes blurred vision, diplopia) | 1.6 | 1.3 |
Events of probable, possible, or uncertain relationship to study drug that occurred in <1.0% of pravastatin-treated patients in the long-term trials included the following; frequencies were similar in placebo-treated patients:
Dermatologic: pruritus, dermatitis, dryness of skin, scalp hair abnormality (including alopecia), urticaria.
Endocrine/Metabolic: sexual dysfunction, libido change.
Gastrointestinal: decreased appetite.
General: fever, flushing.
Immunologic: allergy, edema head/neck.
Musculoskeletal: muscle weakness.
Nervous System: paresthesia, vertigo, insomnia, memory impairment, tremor, neuropathy (including peripheral neuropathy).
Special Senses: lens opacity, taste disturbance.
Postmarketing Experience
In addition to the events reported above, as with other drugs in this class, the following events have been reported rarely during postmarketing experience with pravastatin sodium, regardless of causality assessment:
Musculoskeletal: myopathy, rhabdomyolysis.
Nervous System: dysfunction of certain cranial nerves (including alteration of taste, impairment of extra-ocular movement, facial paresis), peripheral nerve palsy.
Hypersensitivity: anaphylaxis, angioedema, lupus erythematosus-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, hemolytic anemia, positive ANA, ESR increase, arthritis, arthralgia, asthenia, photosensitivity, chills, malaise, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
Gastrointestinal: pancreatitis, hepatitis, including chronic active hepatitis, cholestatic jaundice, fatty change in liver, cirrhosis, fulminant hepatic necrosis, hepatoma.
Dermatologic: a variety of skin changes (e.g., nodules, discoloration, dryness of mucous membranes, changes to hair/nails).
Reproductive: gynecomastia.
Laboratory Abnormalities: Liver Function Test abnormalities, thyroid function abnormalities.
Laboratory Test Abnormalities
Increases in serum transaminase (ALT, AST) values and CPK have been observed (see WARNINGS ).
Transient, asymptomatic eosinophilia has been reported. Eosinophil counts usually returned to normal despite continued therapy. Anemia, thrombocytopenia, and leukopenia have been reported with HMG-CoA reductase inhibitors.
Concomitant Therapy
Pravastatin has been administered concurrently with cholestyramine, colestipol, nicotinic acid, probucol and gemfibrozil. Preliminary data suggest that the addition of either probucol or gemfibrozil to therapy with lovastatin or pravastatin is not associated with greater reduction in LDL-cholesterol than that achieved with lovastatin or pravastatin alone. No adverse reactions unique to the combination or in addition to those previously reported for each drug alone have been reported. Myopathy and rhabdomyolysis (with or without acute renal failure) have been reported when another HMG-CoA reductase inhibitor was used in combination with immunosuppressive drugs, gemfibrozil, erythromycin, or lipid-lowering doses of nicotinic acid. Concomitant therapy with HMG-CoA reductase inhibitors and these agents is generally not recommended. (See WARNINGS: Skeletal Muscle and PRECAUTIONS: Drug interactions .)
Pediatric Patients
In a two-year, double-blind, placebo-controlled study involving 100 boys and 114 girls with HeFH, the safety and tolerability profile of pravastatin was generally similar to that of placebo. (See CLINICAL PHARMACOLOGY: Pediatric Clinical Study and PRECAUTIONS: Pediatric use .)
OVERDOSAGE
To date, there has been limited experience with overdosage of pravastatin. If an overdose occurs, it should be treated symptomatically with laboratory monitoring and supportive measures should be instituted as required. (See WARNINGS .)
PRAVASTATIN SODIUM DOSAGE AND ADMINISTRATION
The patient should be placed on a standard cholesterol-lowering diet before receiving pravastatin sodium and should continue on this diet during treatment with pravastatin sodium (see NCEP Treatment Guidelines for details on dietary therapy).
Pravastatin sodium can be administered orally as a single dose at any time of the day, with or without food. Since the maximal effect of a given dose is seen within 4 weeks, periodic lipid determinations should be performed at this time and dosage adjusted according to the patient’s response to therapy and established treatment guidelines.
Adult Patients
The recommended starting dose is 40 mg once daily. If a daily dose of 40 mg does not achieve desired cholesterol levels, 80 mg once daily is recommended. In patients with a history of significant renal or hepatic dysfunction, a starting dose of 10 mg daily is recommended.
Pediatric Patients
Children (Ages 8 to 13 Years, Inclusive)
The recommended dose is 20 mg once daily in children 8 to 13 years of age. Doses greater than 20 mg have not been studied in this patient population.
Adolescents (Ages 14 to 18 Years)
The recommended starting dose is 40 mg once daily in adolescents 14 to 18 years of age. Doses greater than 40 mg have not been studied in this patient population.
Children and adolescents treated with pravastatin should be reevaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult goals for LDL-C (see INDICATIONS AND USAGE: Hyperlipidemia, NCEP Treatment Guidelines ).
In patients taking immunosuppressive drugs such as cyclosporine (see WARNINGS: Skeletal Muscle ) concomitantly with pravastatin, therapy should begin with 10 mg of pravastatin once-a-day at bedtime and titration to higher doses should be done with caution. Most patients treated with this combination received a maximum pravastatin dose of 20 mg/day.
Concomitant Therapy
The lipid-lowering effects of pravastatin sodium on total and LDL cholesterol are enhanced when combined with a bile-acid-binding resin. When administering a bile-acid-binding resin (e.g., cholestyramine, colestipol) and pravastatin, pravastatin sodium should be given either 1 hour or more before or at least 4 hours following the resin. (See also ADVERSE REACTIONS: Concomitant Therapy .)
HOW SUPPLIED
Pravastatin sodium tablets are supplied as:
40 mg tablets: Green colored, rounded–rectangular shaped tablets having biconvex surface, with “G5” debossed on one surface and “40” debossed on the other surface. They are supplied in bottles of 30 (NDC 42254-131-30) and bottles of 90 (NDC 42254-131-90). Bottles contain a desiccant canister.
STORAGE
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Keep tightly closed (protect from moisture). Protect from light.
REFERENCES
1Shepherd J, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia (WOS). N Engl J Med 1995;333:1301-7.
4Pitt B, et al. Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC I): Reduction in Atherosclerosis Progression and Clinical Events. J Am Coll Cardiol 1995;26:1133-9.
5Jukema JW, et al. Effects of Lipid Lowering by Pravastatin on Progression and Regression of Coronary Artery Disease in Symptomatic Man With Normal to Moderately Elevated Serum Cholesterol Levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation 1995;91:2528-2540.
6Crouse JR, et al. Pravastatin, lipids, and atherosclerosis in the carotid arteries: design features of a clinical trial with carotid atherosclerosis outcome (PLAC II). Controlled Clinical Trials 1992;13:495.
7Salonen R, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Research Institute of Public Health, University of Kuopio, Finland. Circulation 1995;92:1758.
8Fredrickson DS, et al. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N Engl J Med 1967; 276:34-42, 94-102, 148-156, 215-224, 273-281.
9Manson JM, Freyssinges C, Ducrocq MB, Stephenson WP. Postmarketing Surveillance of Lovastatin and Simvastatin Exposure During Pregnancy. ReproductiveToxicology 1996;10(6):439-446.
Manufactured by:
Glenmark Generics Ltd.
Colvale-Bardez, Goa 403 513, India
Manufactured for:

Glenmark Generics Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkgenerics.com
March 2008
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
Principal Display Panel
Pravastatin SodiumPravastatin Sodium TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||