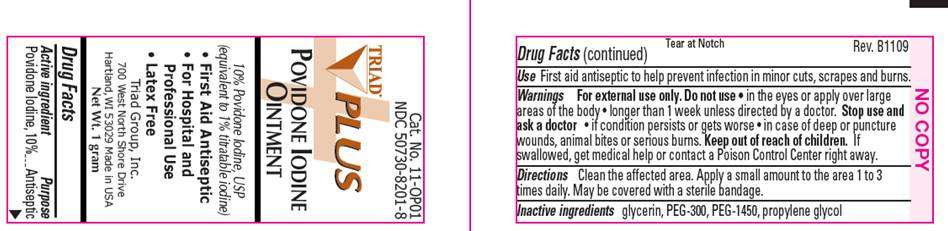
Povidone Iodine Plus
H and P Industries, Inc. dba Triad Group
H and P Industries, Inc. dba Triad Group
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PACKAGE INFORMATION
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
Povidone Iodine USP 10% (equivalent to 1% titratable iodine)
PURPOSE
Antiseptic
USE
WARNINGS
For external use only.
Do not use
- in the eyes or over large areas of the body
- longer than 1 week unless directed by a doctor
Stop use and ask a doctor
- if condition persists or gets worse
- in case of deep puncture wounds, animal bites or serious burns
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
OTHER INFORMATION
INACTIVE INGREDIENTS
PACKAGE INFORMATION
- First Aid Antiseptic
- For Hospital and Professional Use Only
Povidone Iodine Pluspovidone-iodine OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!