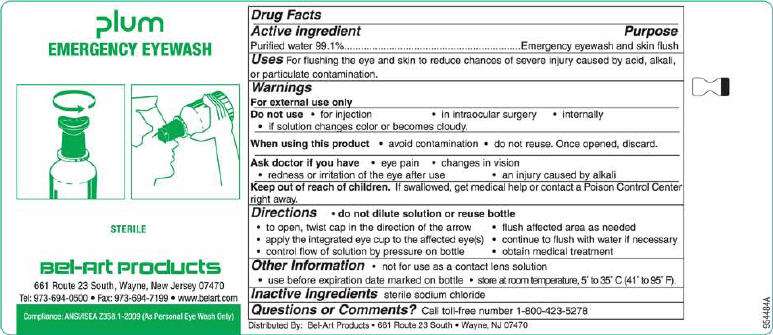
Plum Emergency Eyewash
Plum Emergency Eyewash
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient
Purified water 99.1%
Purpose
Purpose
Emergency eyewash and skin flush
Uses
Uses
For flushing the eye and skin to reduce chances of severe injury caused by acid, alkali, or particulate contamination.
Warnings
For external use only
Do not use
• for injection • in intraocular surgery • internally
• if solution changes color or becomes cloudy.
When using this product • avoid contamination, do not touch tip of container to any surface
• do not reuse. Once opened, discard.
Ask doctor if you have • eye pain • changes in vision
• redness or irritation of the eye after use • an injury caused by alkali
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center
right away.
Directions • do not dilute solution or reuse bottle
• hold container a few inches above the eye or skin
• control flow of solution by pressure on bottle • flush affected area as needed
• continue to flush with water if necessary • obtain medical treatment
Other Information • not for use as a contact lens solution • twist cap to open
• use before expiration date marked on bottle • store at room temperature, 5° to 35° C (41° to 95° F).
Inactive Ingredients sterile sodium chloride
Questions or Comments? Call toll-free number 1-800-423-5278.
Plum
Plum Emergency EyewashWater LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||