Paroxetine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- PAROXETINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PAROXETINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PAROXETINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsAntidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of paroxetine tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Paroxetine is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk,PRECAUTIONS: Information for Patients, andPRECAUTIONS: Pediatric Use.)
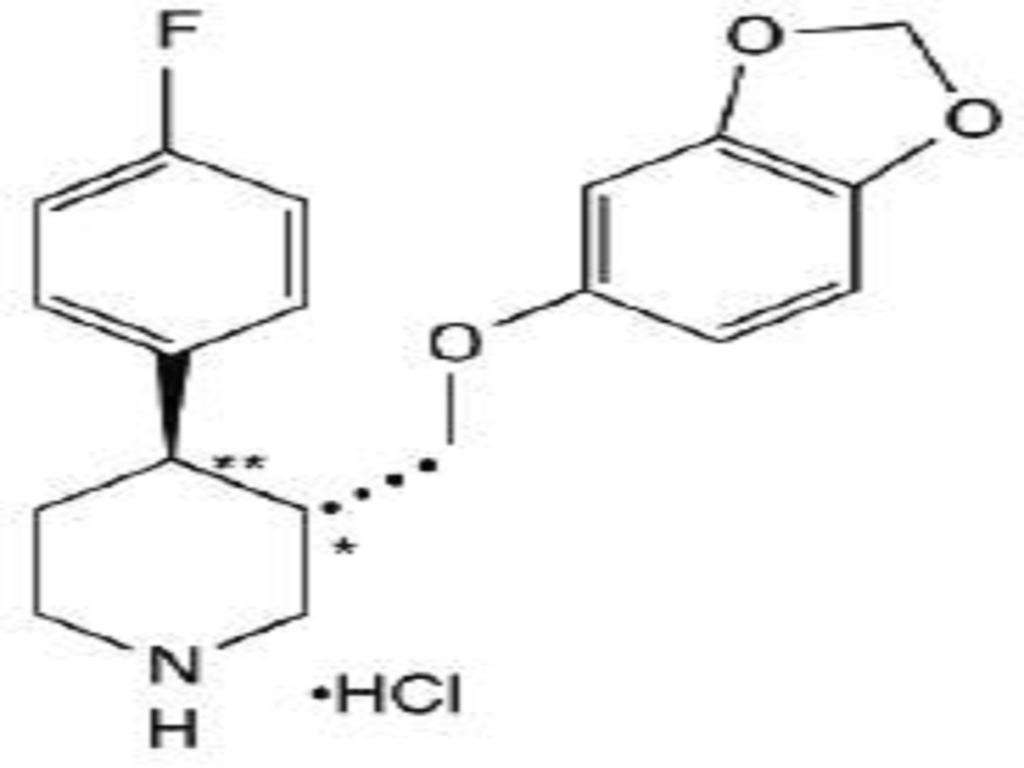
PAROXETINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Pharmacodynamics
Pharmacokinetics
PRECAUTIONS: Drugs Metabolized by CYP2D6
Other Clinical Pharmacology Information
Renal and Liver Disease:
DOSAGE AND ADMINISTRATION
Elderly Patients:DOSAGE AND ADMINISTRATION
PRECAUTIONS: Drug Interactions
Clinical Trials
Outcome Classification (%) on CGI-Global Improvement Item for Completers in Study 1Outcome ClassificationPlacebo (n = 74)Paroxetine 20 mg (n = 75)Paroxetine 40 mg (n = 66)Paroxetine 60 mg (n = 66)
INDICATIONS & USAGE
Major Depressive DisorderCLINICAL PHARMACOLOGY: Clinical Trials
The effects of paroxetine in hospitalized depressed patients have not been adequately studied.
The efficacy of paroxetine in maintaining a response in major depressive disorder for up to 1 year was demonstrated in a placebo-controlled trial (seeCLINICAL PHARMACOLOGY: Clinical Trials). Nevertheless, the physician who elects to use paroxetine for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
Obsessive Compulsive Disorder
Paroxetine tablets, USP are indicated for the treatment of obsessions and compulsions in patients with obsessive compulsive disorder (OCD) as defined in the DSM-IV. The obsessions or compulsions cause marked distress, are time-consuming, or significantly interfere with social or occupational functioning.
The efficacy of paroxetine was established in two 12-week trials with obsessive compulsive outpatients whose diagnoses corresponded most closely to the DSM-IIIR category of obsessive compulsive disorder (seeCLINICAL PHARMACOLOGY: Clinical Trials).
Obsessive compulsive disorder is characterized by recurrent and persistent ideas, thoughts, impulses, or images (obsessions) that are ego-dystonic and/or repetitive, purposeful, and intentional behaviors (compulsions) that are recognized by the person as excessive or unreasonable.
Long-term maintenance of efficacy was demonstrated in a 6-month relapse prevention trial. In this trial, patients assigned to paroxetine showed a lower relapse rate compared to patients on placebo (seeCLINICAL PHARMACOLOGY: Clinical Trials). Nevertheless, the physician who elects to use paroxetine for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (seeDOSAGE AND ADMINISTRATION).
Panic Disorder
Paroxetine tablets, USP are indicated for the treatment of panic disorder, with or without agoraphobia, as defined in DSM-IV. Panic disorder is characterized by the occurrence of unexpected panic attacks and associated concern about having additional attacks, worry about the implications or consequences of the attacks, and/or a significant change in behavior related to the attacks.
The efficacy of paroxetine was established in three 10- to 12-week trials in panic disorder patients whose diagnoses corresponded to the DSM-IIIR category of panic disorder (seeCLINICAL PHARMACOLOGY: Clinical Trials).
Long-term maintenance of efficacy was demonstrated in a 3-month relapse prevention trial. In this trial, patients with panic disorder assigned to paroxetine demonstrated a lower relapse rate compared to patients on placebo (seeCLINICAL PHARMACOLOGY: Clinical Trials). Nevertheless, the physician who prescribes paroxetine for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (seeDOSAGE AND ADMINISTRATION).
Generalized Anxiety Disorder
Paroxetine tablets, USP are indicated for the treatment of Generalized Anxiety Disorder (GAD), as defined in DSM-IV. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The efficacy of paroxetine in the treatment of GAD was established in two 8-week placebo-controlled trials in adults with GAD. Paroxetine has not been studied in children or adolescents with Generalized Anxiety Disorder (seeCLINICAL PHARMACOLOGY: Clinical Trials).
Generalized Anxiety Disorder (DSM-IV) is characterized by excessive anxiety and worry (apprehensive expectation) that is persistent for at least 6 months and which the person finds difficult to control. It must be associated with at least 3 of the following 6 symptoms: Restlessness or feeling keyed up or on edge, being easily fatigued, difficulty concentrating or mind going blank, irritability, muscle tension, sleep disturbance.
The efficacy of paroxetine in maintaining a response in patients with Generalized Anxiety Disorder, who responded during an 8-week acute treatment phase while taking paroxetine and were then observed for relapse during a period of up to 24 weeks, was demonstrated in a placebo-controlled trial (seeCLINICAL PHARMACOLOGY: Clinical Trials). Nevertheless, the physician who elects to use paroxetine for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (seeDOSAGE AND ADMINISTRATION).
PAROXETINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONSPRECAUTIONS
WARNINGS
Clinical Worsening and Suicide Risk
Table 1
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
PRECAUTIONSDOSAGE AND ADMINISTRATION: Discontinuation of Treatment With Paroxetine Tablets
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder
Potential for Interaction With Monoamine Oxidase Inhibitors
In patients receiving another serotonin reuptake inhibitor drug in combination with a monoamine oxidase inhibitor (MAOI), there have been reports of serious, sometimes fatal, reactions including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. These reactions have also been reported in patients who have recently discontinued that drug and have been started on an MAOI. Some cases presented with features resembling neuroleptic malignant syndrome. While there are no human data showing such an interaction with paroxetine, limited animal data on the effects of combined use of paroxetine and MAOIs suggest that these drugs may act synergistically to elevate blood pressure and evoke behavioral excitation. Therefore, it is recommended that paroxetine not be used in combination with an MAOI (including linezolid, an antibiotic which is a reversible non-selective MAOI), or within 14 days of discontinuing treatment with an MAOI (seeCONTRAINDICATIONS). At least 2 weeks should be allowed after stopping paroxetine before starting an MAOI.
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported with SNRIs and SSRIs alone, including treatment with paroxetine, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs which impair metabolism of serotonin (including MAOIs), or with antipsychotics or other dopamine antagonists. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Serotonin syndrome, in its most severe form can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
The concomitant use of paroxetine with MAOIs intended to treat depression is contraindicated.
If concomitant treatment of paroxetine with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases.
The concomitant use of paroxetine with serotonin precursors (such as tryptophan) is not recommended.
Treatment with paroxetine and any concomitant serotonergic or antidopaminergic agents, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Potential Interaction With Thioridazine
Thioridazine administration alone produces prolongation of the QTc interval, which is associated with serious ventricular arrhythmias, such as torsade de pointestype arrhythmias, and sudden death. This effect appears to be dose related.
An in vivo study suggests that drugs which inhibit CYP2D6, such as paroxetine, will elevate plasma levels of thioridazine. Therefore, it is recommended that paroxetine not be used in combination with thioridazine (seeCONTRAINDICATIONSandPRECAUTIONS).
Usage in Pregnancy
Teratogenic Effects
Epidemiological studies have shown that infants born to women who had first trimester paroxetine exposure had an increased risk of cardiovascular malformations, primarily ventricular and atrial septal defects (VSDs and ASDs). In general, septal defects range from those that are symptomatic and may require surgery to those that are asymptomatic and may resolve spontaneously. If a patient becomes pregnant while taking paroxetine, she should be advised of the potential harm to the fetus. Unless the benefits of paroxetine to the mother justify continuing treatment, consideration should be given to either discontinuing paroxetine therapy or switching to another antidepressant (seePRECAUTIONS: Discontinuation of Treatment With Paroxetine Tablets). For women who intend to become pregnant or are in their first trimester of pregnancy, paroxetine should only be initiated after consideration of the other available treatment options.
Animal Findings
Reproduction studies were performed at doses up to 50 mg/kg/day in rats and 6 mg/kg/day in rabbits administered during organogenesis. These doses are approximately 8 (rat) and 2 (rabbit) times the maximum recommended human dose (MRHD) on an mg/m2 basis. These studies have revealed no evidence of teratogenic effects. However, in rats, there was an increase in pup deaths during the first 4 days of lactation when dosing occurred during the last trimester of gestation and continued throughout lactation. This effect occurred at a dose of 1 mg/kg/day or approximately one-sixth of the MRHD on an mg/m2 basis. The no-effect dose for rat pup mortality was not determined. The cause of these deaths is not known.
Nonteratogenic Effects
Neonates exposed to paroxetine and other SSRIs or serotonin and norepinephrine reuptake inhibitors (SNRIs), late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome (seeWARNINGS: Serotonin SyndromePotential for Interaction With Monoamine Oxidase Inhibitors).
There have also been postmarketing reports of premature births in pregnant women exposed to paroxetine or other SSRIs.
When treating a pregnant woman with paroxetine during the third trimester, the physician should carefully consider both the potential risks and benefits of treatment (seeDOSAGE AND ADMINISTRATION). Physicians should note that in a prospective longitudinal study of 201 women with a history of major depression who were euthymic at the beginning of pregnancy, women who discontinued antidepressant medication during pregnancy were more likely to experience a relapse of major depression than women who continued antidepressant medication.
PRECAUTIONS
GeneralDiscontinuation of Treatment With Paroxetine Tablets
DOSAGE AND ADMINISTRATION
PRECAUTIONS: Pediatric Use
Hyponatremia
PRECAUTIONS: Geriatric Use
DOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
Drugs That Interfere With Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
WARNINGS: Usage in Pregnancy: Teratogenic EffectsNonteratogenic Effects
PRECAUTIONS: Nursing Mothers
LABORATORY TESTS
DRUG INTERACTIONS
Tryptophan
WARNINGS: Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
CONTRAINDICATIONSWARNINGS
CONTRAINDICATIONS
WARNINGS: Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like ReactionsCONTRAINDICATIONSPRECAUTIONS: Drug Interactions, Tryptophan
CONTRAINDICATIONSWARNINGS
PRECAUTIONS: Drugs That Interfere With Hemostasis
WARNINGS: Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
ADVERSE REACTIONS: Postmarketing Reports
Drugs Metabolized by CYP2D6
CONTRAINDICATIONSWARNINGS
PRECAUTIONS: Tricyclic Antidepressants (TCAs)
Tricyclic Antidepressants (TCAs)
PRECAUTIONS: Drugs Metabolized by Cytochrome CYP2D6
ADVERSE REACTIONS: Postmarketing Reports
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGS: Usage in Pregnancy: Teratogenic EffectsNonteratogenic EffectsLABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNINGWARNINGS: Clinical Worsening and Suicide Risk
DOSAGE AND ADMINISTRATION: Discontinuation of Treatment With Paroxetine Tablets
GERIATRIC USE
PRECAUTIONS: Hyponatremia
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
PAROXETINE HYDROCHLORIDE ADVERSE REACTIONS
Associated With Discontinuation of TreatmentMajor Depressive DisorderOCDPanic DisorderGeneralized Anxiety DisorderParoxetinePlaceboParoxetinePlaceboParoxetinePlaceboParoxetinePlaceboCommonly Observed Adverse Events
Incidence in Controlled Clinical Trials
Body SystemPreferred TermParoxetine (n=421)Placebo (n=421)
Body SystemPreferred TermObsessive Compulsive DisorderPanic DisorderParoxetine (n = 542)Placebo (n = 265)Paroxetine (n = 469)Placebo (n = 324)
Body SystemPreferred TermGeneralized Anxiety DisorderParoxetine (n=735)Placebo (n=529)
Body System/Preferred TermPlaceboParoxetinen = 5110 mg n = 10220 mg n = 10430 mg n = 10140 mg n = 102
ParoxetinePlacebon (males)14461042
Other Events Observed During the Premarketing Evaluation of Paroxetine
PRECAUTIONS
Postmarketing Reports
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychologic Dependence
OVERDOSAGE
Human ExperienceOverdosage Management
PRECAUTIONS: Drugs Metabolized by Cytochrome CYP2D6
DOSAGE & ADMINISTRATION
Major Depressive DisorderObsessive Compulsive Disorder
CLINICAL PHARMACOLOGY: Clinical Trials
Panic Disorder
CLINICAL PHARMACOLOGY: Clinical Trials
Generalized Anxiety Disorder
CLINICAL PHARMACOLOGY: Clinical Trials
Special Populations
WARNINGS: Usage in Pregnancy
Switching Patients to or From a Monoamine Oxidase Inhibitor
Discontinuation of Treatment With Paroxetine Tablets
PRECAUTIONS: Discontinuation of Treatment With Paroxetine Tablets
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or ActionsParoxetine Tablets, USP
Talk to your, or your family member's, healthcare provider about:
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses.It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects.Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines.Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children.Talk to your child's healthcare provider for more information.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Paroxetine HydrochlorideParoxetine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!