PAROXETINE
STAT Rx USA LLC
PSS World Medical Inc.
Paroxetine Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx only
- Suicidality and Antidepressant Drugs
- PAROXETINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PAROXETINE INDICATIONS AND USAGE
- PAROXETINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PAROXETINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- PAROXETINE DOSAGE AND ADMINISTRATION
- Major Depressive Disorder
- Obsessive Compulsive Disorder
- Panic Disorder
- Generalized Anxiety Disorder
- Special Populations
- Switching Patients to or From a Monoamine Oxidase Inhibitor
- Use of Paroxetine Tablets With Reversible MAOIs Such as Linezolid or Methylene Blue
- Discontinuation of Treatment With Paroxetine Tablets
- HOW SUPPLIED
- Medication Guide
- PACKAGE LABEL - PAROXETINE 20mg TABLETS
FULL PRESCRIBING INFORMATION
Rx only
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of paroxetine tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Paroxetine is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.)
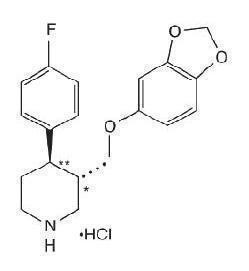
PAROXETINE DESCRIPTION
transRS192032
CLINICAL PHARMACOLOGY
Pharmacodynamics
The efficacy of paroxetine in the treatment of major depressive disorder, obsessive compulsive disorder (OCD), panic disorder (PD), and generalized anxiety disorder (GAD) is presumed to be linked to potentiation of serotonergic activity in the central nervous system resulting from inhibition of neuronal reuptake of serotonin (5-hydroxy-tryptamine, 5-HT). Studies at clinically relevant doses in humans have demonstrated that paroxetine blocks the uptake of serotonin into human platelets. In vitro studies in animals also suggest that paroxetine is a potent and highly selective inhibitor of neuronal serotonin reuptake and has only very weak effects on norepinephrine and dopamine neuronal reuptake. In vitro radioligand binding studies indicate that paroxetine has little affinity for muscarinic, alpha1-, alpha2-, beta-adrenergic-, dopamine (D2)-, 5-HT1-, 5-HT2-, and histamine (H1)-receptors; antagonism of muscarinic, histaminergic, and alpha1-adrenergic receptors has been associated with various anticholinergic, sedative, and cardiovascular effects for other psychotropic drugs.
Because the relative potencies of paroxetine’s major metabolites are at most 1/50 of the parent compound, they are essentially inactive.
Pharmacokinetics
Paroxetine hydrochloride is completely absorbed after oral dosing of a solution of the hydrochloride salt. The mean elimination half-life is approximately 21 hours (CV 32%) after oral dosing of 30 mg tablets of paroxetine daily for 30 days. Paroxetine is extensively metabolized and the metabolites are considered to be inactive. Nonlinearity in pharmacokinetics is observed with increasing doses. Paroxetine metabolism is mediated in part by CYP2D6, and the metabolites are primarily excreted in the urine and to some extent in the feces. Pharmacokinetic behavior of paroxetine has not been evaluated in subjects who are deficient in CYP2D6 (poor metabolizers).
In a meta-analysis of paroxetine from 4 studies done in healthy volunteers following multiple dosing of 20 mg/day to 40 mg/day, males did not exhibit a significantly lower Cmax or AUC than females.
Absorption and Distribution
Paroxetine hydrochloride is completely absorbed after oral dosing of a solution of the hydrochloride salt. In a study in which normal male subjects (n = 15) received 30 mg tablets daily for 30 days, steady-state paroxetine concentrations were achieved by approximately 10 days for most subjects, although it may take substantially longer in an occasional patient. At steady state, mean values of Cmax, Tmax,Cmin, and T½ were 61.7 ng/mL (CV 45%), 5.2 hr. (CV 10%), 30.7 ng/mL (CV 67%), and 21 hours (CV 32%), respectively. The steady-state Cmax and Cmin values were about 6 and 14 times what would be predicted from single-dose studies. Steady-state drug exposure based on AUC0-24 was about 8 times greater than would have been predicted from single-dose data in these subjects. The excess accumulation is a consequence of the fact that 1 of the enzymes that metabolizes paroxetine is readily saturable.
The effects of food on the bioavailability of paroxetine were studied in subjects administered a single dose with and without food. AUC was only slightly increased (6%) when drug was administered with food but the Cmax was 29% greater, while the time to reach peak plasma concentration decreased from 6.4 hours post-dosing to 4.9 hours.
Paroxetine distributes throughout the body, including the CNS, with only 1% remaining in the plasma.
Approximately 95% and 93% of paroxetine is bound to plasma protein at 100 ng/mL and 400 ng/mL, respectively. Under clinical conditions, paroxetine concentrations would normally be less than 400 ng/mL. Paroxetine does not alter the in vitro protein binding of phenytoin or warfarin.
Metabolism and Excretion
The mean elimination half-life is approximately 21 hours (CV 32%) after oral dosing of 30 mg tablets daily for 30 days of paroxetine. In steady-state dose proportionality studies involving elderly and nonelderly patients, at doses of 20 mg to 40 mg daily for the elderly and 20 mg to 50 mg daily for the nonelderly, some nonlinearity was observed in both populations, again reflecting a saturable metabolic pathway. In comparison to Cmin values after 20 mg daily, values after 40 mg daily were only about 2 to 3 times greater than doubled.
PRECAUTIONS: Drugs Metabolized by CYP2D6Other Clinical Pharmacology Information
Specific Populations
Renal and Liver Disease: Increased plasma concentrations of paroxetine occur in subjects with renal and hepatic impairment. The mean plasma concentrations in patients with creatinine clearance below 30 mL/min. were approximately 4 times greater than seen in normal volunteers. Patients with creatinine clearance of 30 to 60 mL/min. and patients with hepatic functional impairment had about a 2-fold increase in plasma concentrations (AUC, Cmax).
DOSAGE AND ADMINISTRATION
Elderly Patients: minmin DOSAGE AND ADMINISTRATION
Drug-Drug Interactions
In vitro PRECAUTIONS: Drug Interactions
Clinical Trials
Major Depressive Disorder
Obsessive Compulsive Disorder
| for Completers in Study 1 | ||||
|---|---|---|---|---|
| Outcome Classification |
Placebo (n = 74) |
Paroxetine 20 mg (n = 75) |
Paroxetine 40 mg (n = 66) |
Paroxetine 60 mg (n = 66) |
| Worse |
14% |
7% |
7% |
3% |
| No Change |
44% |
35% |
22% |
19% |
| Minimally Improved |
24% |
33% |
29% |
34% |
| Much Improved |
11% |
18% |
22% |
24% |
| Very Much Improved |
7% |
7% |
20% |
20% |
Panic Disorder
The effectiveness of paroxetine in the treatment of panic disorder was demonstrated in three 10- to 12-week multicenter, placebo-controlled studies of adult outpatients (Studies 1 to 3). Patients in all studies had panic disorder (DSM-IIIR), with or without agoraphobia. In these studies, paroxetine was shown to be significantly more effective than placebo in treating panic disorder by at least 2 out of 3 measures of panic attack frequency and on the Clinical Global Impression Severity of Illness score.
Study 1 was a 10-week dose-range finding study; patients were treated with fixed paroxetine doses of 10, 20, or 40 mg/day or placebo. A significant difference from placebo was observed only for the 40 mg/day group. At endpoint, 76% of patients receiving paroxetine 40 mg/day were free of panic attacks, compared to 44% of placebo-treated patients.
Study 2 was a 12-week flexible-dose study comparing paroxetine (10 to 60 mg daily) and placebo. At endpoint, 51% of paroxetine patients were free of panic attacks compared to 32% of placebo-treated patients.
Study 3 was a 12-week flexible-dose study comparing paroxetine (10 to 60 mg daily) to placebo in patients concurrently receiving standardized cognitive behavioral therapy. At endpoint, 33% of the paroxetine-treated patients showed a reduction to 0 or 1 panic attacks compared to 14% of placebo patients.
In both Studies 2 and 3, the mean paroxetine dose for completers at endpoint was approximately 40 mg/day of paroxetine.
Generalized Anxiety Disorder
The effectiveness of paroxetine in the treatment of Generalized Anxiety Disorder (GAD) was demonstrated in two 8-week, multicenter, placebo-controlled studies (Studies 1 and 2) of adult outpatients with Generalized Anxiety Disorder (DSM-IV).
Study 1 was an 8-week study comparing fixed paroxetine doses of 20 mg or 40 mg/day with placebo. Doses of 20 mg or 40 mg of paroxetine were both demonstrated to be significantly superior to placebo on the Hamilton Rating Scale for Anxiety (HAM-A) total score. There was not sufficient evidence in this study to suggest a greater benefit for the 40 mg/day dose compared to the 20 mg/day dose.
Study 2 was a flexible-dose study comparing paroxetine (20 mg to 50 mg daily) and placebo. Paroxetine demonstrated statistically significant superiority over placebo on the Hamilton Rating Scale for Anxiety (HAM-A) total score. A third study, also flexible-dose comparing paroxetine (20 mg to 50 mg daily), did not demonstrate statistically significant superiority of paroxetine over placebo on the Hamilton Rating Scale for Anxiety (HAM-A) total score, the primary outcome.
PAROXETINE INDICATIONS AND USAGE
Major Depressive Disorder
Paroxetine tablets, USP are indicated for the treatment of major depressive disorder.
The efficacy of paroxetine in the treatment of a major depressive episode was established in 6-week controlled trials of outpatients whose diagnoses corresponded most closely to the DSM-III category of major depressive disorder (see CLINICAL PHARMACOLOGY: Clinical Trials ). A major depressive episode implies a prominent and relatively persistent depressed or dysphoric mood that usually interferes with daily functioning (nearly every day for at least 2 weeks); it should include at least 4 of the following 8 symptoms: Change in appetite, change in sleep, psychomotor agitation or retardation, loss of interest in usual activities or decrease in sexual drive, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, and a suicide attempt or suicidal ideation.
CLINICAL PHARMACOLOGY: Clinical Trials
Obsessive Compulsive Disorder
Paroxetine tablets, USP are indicated for the treatment of obsessions and compulsions in patients with obsessive compulsive disorder (OCD) as defined in the DSM-IV. The obsessions or compulsions cause marked distress, are time-consuming, or significantly interfere with social or occupational functioning.
The efficacy of paroxetine was established in two 12-week trials with obsessive compulsive outpatients whose diagnoses corresponded most closely to the DSM-IIIR category of obsessive compulsive disorder (see CLINICAL PHARMACOLOGY: Clinical Trials ).
CLINICAL PHARMACOLOGY: Clinical Trials DOSAGE AND ADMINISTRATION
Panic Disorder
Paroxetine tablets, USP are indicated for the treatment of panic disorder, with or without agoraphobia, as defined in DSM-IV. Panic disorder is characterized by the occurrence of unexpected panic attacks and associated concern about having additional attacks, worry about the implications or consequences of the attacks, and/or a significant change in behavior related to the attacks.
The efficacy of paroxetine was established in three 10- to 12-week trials in panic disorder patients whose diagnoses corresponded to the DSM-IIIR category of panic disorder (see CLINICAL PHARMACOLOGY:Clinical Trials ).
CLINICAL PHARMACOLOGY:Clinical Trials DOSAGE AND ADMINISTRATION
Generalized Anxiety Disorder
Paroxetine tablets, USP are indicated for the treatment of Generalized Anxiety Disorder (GAD), as defined in DSM-IV. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The efficacy of paroxetine in the treatment of GAD was established in two 8-week placebo-controlled trials in adults with GAD. Paroxetine has not been studied in children or adolescents with Generalized Anxiety Disorder (see CLINICAL PHARMACOLOGY: Clinical Trials ).
CLINICAL PHARMACOLOGY: Clinical Trials DOSAGE AND ADMINISTRATION
PAROXETINE CONTRAINDICATIONS
WARNINGS
WARNINGS
WARNINGS PRECAUTIONS
PRECAUTIONS
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
|
Table 1 |
|
| Age Range |
Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Patients Treated |
| Increases Compared to Placebo |
|
| <18 |
14 additional cases |
| 18-24 |
5 additional cases |
| Decreases Compared to Placebo |
|
| 25-64 |
1 fewer case |
| ≥65 |
6 fewer cases |
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
PRECAUTIONS DOSAGE AND ADMINISTRATION:Discontinuation of Treatment with Paroxetine Tablets
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder
Potential for Interaction With Monoamine Oxidase Inhibitors
CONTRAINDICATIONSDOSAGE AND ADMINISTRATION
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported with SNRIs and SSRIs alone, including treatment with paroxetine, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs which impair metabolism of serotonin (including MAOIs), or with antipsychotics or other dopamine antagonists. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Serotonin syndrome, in its most severe form can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
The concomitant use of paroxetine with MAOIs intended to treat depression is contraindicated.
If concomitant treatment of paroxetine with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases.
The concomitant use of paroxetine with serotonin precursors (such as tryptophan) is not recommended.
Treatment with paroxetine and any concomitant serotonergic or antidopaminergic agents, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Potential Interaction With Thioridazine
Thioridazine administration alone produces prolongation of the QTc interval, which is associated with serious ventricular arrhythmias, such as torsade de pointestype arrhythmias, and sudden death. This effect appears to be dose related.
An in vivo study suggests that drugs which inhibit CYP2D6, such as paroxetine, will elevate plasma levels of thioridazine. Therefore, it is recommended that paroxetine not be used in combination with thioridazine (see CONTRAINDICATIONS and PRECAUTIONS).
Usage in Pregnancy
Teratogenic Effects
Epidemiological studies have shown that infants exposed to paroxetine in the first trimester of pregnancy have an increased risk of congenital malformations, particularly cardiovascular malformations. The findings from these studies are summarized below:
- A study based on Swedish national registry data demonstrated that infants exposed to paroxetine during pregnancy (n = 815) had an increased risk of cardiovascular malformations (2% risk in paroxetine-exposed infants) compared to the entire registry population (1% risk), for an odds ratio (OR) of 1.8 (95% confidence interval 1.1 to 2.8). No increase in the risk of overall congenital malformations was seen in the paroxetine-exposed infants. The cardiac malformations in the paroxetine-exposed infants were primarily ventricular septal defects (VSDs) and atrial septal defects (ASDs). Septal defects range in severity from those that resolve spontaneously to those which require surgery.
- A separate retrospective cohort study from the United States (United Healthcare data) evaluated 5,956 infants of mothers dispensed antidepressants during the first trimester (n = 815 for paroxetine). This study showed a trend towards an increased risk for cardiovascular malformations for paroxetine (risk of 1.5%) compared to other antidepressants (risk of 1%), for an OR of 1.5 (95% confidence interval 0.8 to 2.9). Of the 12 paroxetine-exposed infants with cardiovascular malformations, 9 had VSDs. This study also suggested an increased risk of overall major congenital malformations including cardiovascular defects for paroxetine (4% risk) compared to other (2% risk) antidepressants (OR 1.8; 95% confidence interval 1.2 to 2.8).
- Two large case-control studies using separate databases, each with >9,000 birth defect cases and >4,000 controls, found that maternal use of paroxetine during the first trimester of pregnancy was associated with a 2- to 3-fold increased risk of right ventricular outflow tract obstructions. In one study the odds ratio was 2.5 (95% confidence interval, 1 to 6, 7 exposed infants) and in the other study the odds ratio was 3.3 (95% confidence interval, 1.3 to 8.8, 6 exposed infants).
Other studies have found varying results as to whether there was an increased risk of overall, cardiovascular, or specific congenital malformations. A meta-analysis of epidemiological data over a 16-year period (1992 to 2008) on first trimester paroxetine use in pregnancy and congenital malformations included the above-noted studies in addition to others (n = 17 studies that included overall malformations and n = 14 studies that included cardiovascular malformations; n = 20 distinct studies). While subject to limitations, this meta-analysis suggested an increased occurrence of cardiovascular malformations (prevalence odds ratio [POR] 1.5; 95% confidence interval 1.2 to 1.9) and overall malformations (POR 1.2; 95% confidence interval 1.1 to 1.4) with paroxetine use during the first trimester. It was not possible in this meta-analysis to determine the extent to which the observed prevalence of cardiovascular malformations might have contributed to that of overall malformations, nor was it possible to determine whether any specific types of cardiovascular malformations might have contributed to the observed prevalence of all cardiovascular malformations.
If a patient becomes pregnant while taking paroxetine, she should be advised of the potential harm to the fetus. Unless the benefits of paroxetine to the mother justify continuing treatment, consideration should be given to either discontinuing paroxetine therapy or switching to another antidepressant (see PRECAUTIONS: Discontinuation of Treatment With Paroxetine Tablets). For women who intend to become pregnant or are in their first trimester of pregnancy, paroxetine should only be initiated after consideration of the other available treatment options.
Animal Findings
22
Nonteratogenic Effects
WARNINGS : Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions ).
th
DOSAGE AND ADMINISTRATION
PRECAUTIONS
General
Activation of Mania/Hypomania
Seizures
Discontinuation of Treatment With Paroxetine Tablets
Recent clinical trials supporting the various approved indications for paroxetine employed a taper-phase regimen, rather than an abrupt discontinuation of treatment. The taper-phase regimen used in GAD clinical trials involved an incremental decrease in the daily dose by 10 mg/day at weekly intervals. When a daily dose of 20 mg/day was reached, patients were continued on this dose for 1 week before treatment was stopped.
With this regimen in those studies, the following adverse events were reported at an incidence of 2% or greater for paroxetine and were at least twice that reported for placebo: Abnormal dreams, paresthesia, and dizziness. In the majority of patients, these events were mild to moderate and were self-limiting and did not require medical intervention.
During marketing of paroxetine and other SSRIs and SNRIs, there have been spontaneous reports of adverse events occurring upon the discontinuation of these drugs (particularly when abrupt), including the following: Dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias such as electric shock sensations and tinnitus), anxiety, confusion, headache, lethargy, emotional lability, insomnia, and hypomania. While these events are generally self-limiting, there have been reports of serious discontinuation symptoms.
DOSAGE AND ADMINISTRATION
PRECAUTIONS: Pediatric Use
Tamoxifen
Some studies have shown that the efficacy of tamoxifen, as measured by the risk of breast cancer relapse/mortality, may be reduced when co-prescribed with paroxetine as a result of paroxetine’s irreversible inhibition of CYP2D6 (see Drug Interactions ). However, other studies have failed to demonstrate such a risk. It is uncertain whether the coadministration of paroxetine and tamoxifen has a significant adverse effect on the efficacy of tamoxifen. One study suggests that the risk may increase with longer duration of coadministration. When tamoxifen is used for the treatment or prevention of breast cancer, prescribers should consider using an alternative antidepressant with little or no CYP2D6 inhibition.
Akathisia
Hyponatremia
PRECAUTIONS: Geriatric Use
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
Abnormal Bleeding
Bone Fracture
Epidemiological studies on bone fracture risk following exposure to some antidepressants, including SSRIs, have reported an association between antidepressant treatment and fractures. There are multiple possible causes for this observation and it is unknown to what extent fracture risk is directly attributable to SSRI treatment. The possibility of a pathological fracture, that is, a fracture produced by minimal trauma in a patient with decreased bone mineral density, should be considered in patients treated with paroxetine who present with unexplained bone pain, point tenderness, swelling, or bruising.
Use in Patients With Concomitant Illness
Clinical experience with paroxetine in patients with certain concomitant systemic illness is limited. Caution is advisable in using paroxetine in patients with diseases or conditions that could affect metabolism or hemodynamic responses.
As with other SSRIs, mydriasis has been infrequently reported in premarketing studies with paroxetine. A few cases of acute angle closure glaucoma associated with paroxetine therapy have been reported in the literature. As mydriasis can cause acute angle closure in patients with narrow angle glaucoma, caution should be used when paroxetine is prescribed for patients with narrow angle glaucoma.
DOSAGE AND ADMINISTRATION
Information for Patients
Paroxetine should not be chewed or crushed, and should be swallowed whole.
Patients should be cautioned about the risk of serotonin syndrome with the concomitant use of paroxetine and triptans, tramadol, or other serotonergic agents.
Clinical Worsening and Suicide Risk
Drugs That Interfere With Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Interference With Cognitive and Motor Performance
Completing Course of Therapy
Concomitant Medication
Alcohol
Pregnancy
WARNINGS: Usage in Pregnancy: Teratogenic and Nonteratogenic Effects
Nursing
PRECAUTIONS: Nursing Mothers
Laboratory Tests
Drug Interactions
Tryptophan
WARNINGS: Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions).
Monoamine Oxidase Inhibitors
CONTRAINDICATIONS WARNINGS
Pimozide
maxmax CONTRAINDICATIONS
Serotonergic Drugs
WARNINGS: Serotonin Syndrome of Neuroleptic Malignant Syndrome (NMS)-like Reactions)
CONTRAINDICATIONS PRECAUTIONS: Drug Interactions: Tryptophan
Thioridazine
CONTRAINDICATIONS WARNINGS
Warfarin
PRECAUTIONS: Drugs That Interfere With Hemostasis
Triptans
WARNINGS: Seratonin Syndrome or Neuroleptic Malignant Syndrome (NMS) - like reactions
Drugs Affecting Hepatic Metabolism
Cimetidine
450
Phenobarbital
450½
Phenytoin
½ ADVERSE REACTIONS: Postmarketing Reports
Drugs Metabolized by CYP2D6
Many drugs, including most drugs effective in the treatment of major depressive disorder (paroxetine, other SSRIs and many tricyclics), are metabolized by the cytochrome P450 isozyme CYP2D6. Like other agents that are metabolized by CYP2D6, paroxetine may significantly inhibit the activity of this isozyme. In most patients (>90%), this CYP2D6 isozyme is saturated early during dosing with paroxetine. In 1 study, daily dosing of paroxetine (20 mg once daily) under steady-state conditions increased single dose desipramine (100 mg) Cmax, AUC, and T½ by an average of approximately 2-, 5-, and 3-fold, respectively. Concomitant use of paroxetine with risperidone, a CYP2D6 substrate has also been evaluated. In 1 study, daily dosing of paroxetine 20 mg in patients stabilized on risperidone (4 to 8 mg/day) increased mean plasma concentrations of risperidone approximately 4-fold, decreased 9-hydroxyrisperidone concentrations approximately 10%, and increased concentrations of the active moiety (the sum of risperidone plus 9-hydroxyrisperidone) approximately 1.4-fold. The effect of paroxetine on the pharmacokinetics of atomoxetine has been evaluated when both drugs were at steady state. In healthy volunteers who were extensive metabolizers of CYP2D6, paroxetine 20 mg daily was given in combination with 20 mg atomoxetine every 12 hours. This resulted in increases in steady state atomoxetine AUC values that were 6- to 8-fold greater and in atomoxetine Cmax values that were 3- to 4-fold greater than when atomoxetine was given alone. Dosage adjustment of atomoxetine may be necessary and it is recommended that atomoxetine be initiated at a reduced dose when it is given with paroxetine.
Concomitant use of paroxetine with other drugs metabolized by cytochrome CYP2D6 has not been formally studied but may require lower doses than usually prescribed for either paroxetine or the other drug.
Therefore, coadministration of paroxetine with other drugs that are metabolized by this isozyme, including certain drugs effective in the treatment of major depressive disorder (e.g., nortriptyline, amitriptyline, imipramine, desipramine, and fluoxetine), phenothiazines, risperidone, and Type 1C antiarrhythmics (e.g., propafenone, flecainide, and encainide), or that inhibit this enzyme (e.g., quinidine), should be approached with caution.
However, due to the risk of serious ventricular arrhythmias and sudden death potentially associated with elevated plasma levels of thioridazine, paroxetine and thioridazine should not be coadministered (see CONTRAINDICATIONS and WARNINGS ).
PRECAUTIONS
450 PRECAUTIONS: Tricyclic Antidepressants (TCAs)).
Drugs Metabolized by Cytochrome CYP3A4
in vivoin vitroin vitroiin vivo
Tricyclic Antidepressants (TCAs)
PRECAUTIONS: Drugs Metabolized by Cytochrome CYP2D6
Drugs Highly Bound to Plasma Protein
Drugs That Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Alcohol
Lithium
Digoxin
Diazepam
Procyclidine
0-24maxmin
Beta-Blockers
ADVERSE REACTIONS: Postmarketing Reports
Theophylline
Fosamprenavir/Ritonavir
Electroconvulsive Therapy (ECT)
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Carcinogenesis
2
Mutagenesis
in vitroin vivoin vivoin vitro
Impairment of Fertility
Some clinical studies have shown that SSRIs (including paroxetine) may affect sperm quality during SSRI treatment, which may affect fertility in some men.
22Pregnancy
WARNINGS: Usage in Pregnancy: Teratogenic Effects and Nonteratogenic Effects.
Labor & Delivery
Nursing Mothers
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS: Clinical Worsening and Suicide Risk ). Three placebo-controlled trials in 752 pediatric patients with MDD have been conducted with paroxetine, and the data were not sufficient to support a claim for use in pediatric patients. Anyone considering the use of paroxetine in a child or adolescent must balance the potential risks with the clinical need. Decreased appetite and weight loss have been observed in association with the use of SSRIs. Consequently, regular monitoring of weight and growth should be performed in children and adolescents treated with an SSRI such as paroxetine.
DOSAGE AND ADMINISTRATION: Discontinuation of Treatment With Paroxetine Tablets
Geriatric Use
PRECAUTIONS: Hyponatremia
CLINICAL PHARMACOLOGY DOSAGE AND ADMINISTRATION
PAROXETINE ADVERSE REACTIONS
Associated With Discontinuation of Treatment
| Major Depressive Disorder |
OCD | Panic Disorder | Generalized Anxiety Disorder |
|||||
|---|---|---|---|---|---|---|---|---|
| Paroxetine | Placebo | Paroxetine | Placebo | Paroxetine | Placebo | Paroxetine | Placebo | |
| Where numbers are not provided the incidence of the adverse events in patients treated with paroxetine was not >1% or was not greater than or equal to 2 times the incidence of placebo. 1. Incidence corrected for gender. |
||||||||
|
CNS
Somnolence Insomnia Agitation Tremor Anxiety Dizziness |
2.3% — 1.1% 1.1% — — |
0.7% — 0.5% 0.3% — — |
— 1.7% — — — 1.5% |
0% 0% |
1.9% 1.3% |
0.3% 0.3% |
2% 1% |
0.2% 0.2% |
|
Gastrointestinal
Constipation Nausea Diarrhea Dry mouth Vomiting Flatulence |
— 3.2% 1% 1% 1% |
1.1% 0.3% 0.3% 0.3% |
1.1% 1.9% — — — |
0% 0% |
3.2% |
1.2% |
2% |
0.2% |
|
Other Asthenia Abnormal Ejaculation1 Sweating Impotence1 |
1.6% 1.6% 1% — |
0.4% 0% 0.3% |
1.9% 2.1% — 1.5% |
0.4% 0% 0% |
|
|
1.8% 2.5% 1.1% |
0.2% 0.5% 0.2% |
| Libido Decreased |
|
|
|
|
|
|
|
|
Commonly Observed Adverse Events
Major Depressive Disorder
Obsessive Compulsive Disorder
Panic Disorder
Generalized Anxiety Disorder
Incidence in Controlled Clinical Trials
Major Depressive Disorder
Table 2. Treatment-Emergent Adverse Experience Incidence in Placebo-Controlled Clinical Trials for Major Depressive Disorder1
| Body System | Preferred Term | Paroxetine (n=421) |
Placebo (n=421) |
|---|---|---|---|
| 1. Events reported by at least 1% of patients treated with paroxetine are included, except the following events which had an incidence on placebo ≥ paroxetine: Abdominal pain, agitation, back pain, chest pain, CNS stimulation, fever, increased appetite, myoclonus, pharyngitis, postural hypotension, respiratory disorder (includes mostly “cold symptoms” or “URI”), trauma, and vomiting. 2. Includes mostly “lump in throat” and “tightness in throat.” 3. Percentage corrected for gender. 4. Mostly “ejaculatory delay.” 5. Includes “anorgasmia,” “erectile difficulties,” “delayed ejaculation/orgasm,” and “sexual dysfunction,” and “impotence.” 6. Includes mostly “difficulty with micturition” and “urinary hesitancy.” 7. Includes mostly “anorgasmia” and “difficulty reaching climax/orgasm.” |
|||
|
Body as a Whole |
Headache Asthenia |
18% 15% |
17% 6% |
| Cardiovascular |
Palpitation Vasodilation |
3% 3% |
1% 1% |
| Dermatologic |
Sweating Rash |
11% 2% |
2% 1% |
| Gastrointestinal |
Nausea Dry Mouth Constipation Diarrhea Decreased Appetite Flatulence Oropharynx Disorder2 Dyspepsia |
26% 18% 14% 12% 6% 4% 2% 2% |
9% 12% 9% 8% 2% 2% 0% 1% |
| Musculoskeletal |
Myopathy Myalgia Myasthenia |
2% 2% 1% |
1% 1% 0% |
| Nervous System |
Somnolence Dizziness Insomnia Tremor Nervousness Anxiety Paresthesia Libido Decreased Drugged Feeling Confusion |
23% 13% 13% 8% 5% 5% 4% 3% 2% 1% |
9% 6% 6% 2% 3% 3% 2% 0% 1% 0% |
| Respiration |
Yawn |
4% |
0% |
| Special Senses |
Blurred Vision Taste Perversion |
4% 2% |
1% 0% |
| Urogenital System |
Ejaculatory Disturbance3,4
Other Male Genital Disorders3,5 Urinary Frequency Urination Disorder6 Female Genital Disorders3,7 |
13% 10% 3% 3% 2% |
0% 0% 1% 0% 0% |
Obsessive Compulsive Disorder and Panic Disorder
Table 3. Treatment-Emergent Adverse Experience Incidence in Placebo-Controlled Clinical Trials for Obsessive Compulsive Disorder and Panic Disorder1
| Body System | Preferred Term | Obsessive Compulsive Disorder |
Panic Disorder | ||
|---|---|---|---|---|---|
| Paroxetine (n=542) |
Placebo (n=265) |
Paroxetine (n=469) |
Placebo (n=324) |
||
| Body as a Whole |
Asthenia Abdominal Pain Chest Pain Back Pain Chills Trauma |
22% — 3% — 2% — |
14% — 2% — 1% — |
14% 4% — 3% 2% — |
5% 3% — 2% 1% — |
| Cardiovascular |
Vasodilation Palpitation |
4% 2% |
1% 0% |
— — |
— — |
| Dermatologic |
Sweating Rash |
9% 3% |
3% 2% |
14% — |
6% — |
| Gastrointestinal |
Nausea Dry Mouth |
23% 18% 16% |
10% 9% 6% |
23% 18% 8% |
17% 11% 5% |
| |
Constipation |
||||
| |
Diarrhea Decreased Appetite Dyspepsia Flatulence Increased Appetite Vomiting |
10% 9% — — 4% — |
10% 3% — — 3% — |
12% 7% — — 2% — |
7% 3% — — 1% — |
| Musculoskeletal |
Myalgia |
— |
— |
— |
— |
| Nervous System |
Insomnia Somnolence Dizziness Tremor Nervousness Libido Decreased Agitation Anxiety Abnormal Dreams Concentration Impaired Depersonalization Myoclonus Amnesia |
24% 24% 12% 11% 9% 7% — — 4% 3% 3% 3% 2% |
13% 7% 6% 1% 8% 4% — — 1% 2% 0% 0% 1% |
18% 19% 14% 9% — 9% 5% 5% — — — 3% — |
10% 11% 10% 1% — 1% 4% 4% — — — 2% — |
| Respiratory System |
Rhinitis Pharyngitis Yawn |
— — — |
— — — |
3% — — |
0% — — |
| Special Senses |
Abnormal Vision Taste Perversion |
4% 2% |
2% 0% |
— — |
— — |
| Urogenital System |
Abnormal Ejaculation2 Dysmenorrhea Female Genital Disorder2 Impotence2 Urinary Frequency Urination Impaired Urinary Tract Infection |
23% — 3% 8% 3% 3% 2% |
1% — 0% 1% 1% 0% 1% |
21% — 9% 5% 2% — 2% |
1% — 1% 0% 0% — 1% |
|
1. Events reported by at least 2% of OCD and panic disorder in patients treated with paroxetine are included, except the following events which had an incidence on placebo ≥ paroxetine: [OCD]: Abdominal pain, agitation, anxiety, back pain, cough increased, depression, headache, hyperkinesia, infection, paresthesia, pharyngitis, respiratory disorder, rhinitis, and sinusitis. [panic disorder]: Abnormal dreams, abnormal vision, chest pain, cough increased, depersonalization, depression, dysmenorrhea, dyspepsia, flu syndrome, headache, infection, myalgia, nervousness, palpitation, paresthesia, pharyngitis, rash, respiratory disorder, sinusitis, taste perversion, trauma, urination impaired, and vasodilation. 2. Percentage corrected for gender. |
|||||
Generalized Anxiety Disorder
Table 4. Treatment-Emergent Adverse Experience Incidence in Placebo-Controlled Clinical Trials for Generalized Anxiety Disorder1
| Body System | Preferred Term | Generalized Anxiety Disorder | |
|---|---|---|---|
| Paroxetine (n=735) |
Placebo (n=529) |
||
| Body as a Whole |
Asthenia Headache Infection Abdominal Pain Trauma |
14% 17% 6% |
6% 14% 3% |
| Cardiovascular |
Vasodilation |
3% |
1% |
| Dermatologic |
Sweating |
6% |
2% |
| Gastrointestinal |
Nausea Dry Mouth Constipation Diarrhea Decreased Appetite Vomiting Dyspepsia |
20% 11% 10% 9% 5% 3% — |
5% 5% 2% 7% 1% 2% — |
| Nervous System |
Insomnia Somnolence Dizziness Tremor Nervousness Libido Decreased Abnormal Dreams |
11% 15% 6% 5% 4% 9% |
8% 5% 5% 1% 3% 2% |
| Respiratory System |
Respiratory Disorder Sinusitis Yawn |
7% 4% 4% |
5% 3% — |
| Special Senses |
Abnormal Vision |
2% |
1% |
| Urogenital System |
Abnormal Ejaculation2
Female Genital Disorder2 Impotence2 |
25% 4% 4% |
2% 1% 3% |
|
1. Events reported by at least 2% of GAD in patients treated with paroxetine are included, except the following events which had an incidence on placebo ≥ paroxetine [GAD]: Abdominal pain, back pain, trauma, dyspepsia, myalgia, and pharyngitis. 2. Percentage corrected for gender. |
|||
Dose Dependency of Adverse Events
Table 5. Treatment-Emergent Adverse Experience Incidence in a Dose-Comparison Trial in the Treatment of Major Depressive Disorder*
| Body System/Preferred Term | Placebo | Paroxetine | |||
|---|---|---|---|---|---|
| n=51 | 10 mg n=102 |
20 mg n=104 |
30 mg n=101 |
40 mg n=102 |
|
|
* Rule for including adverse events in table: Incidence at least 5% for 1 of paroxetine groups and ≥ twice the placebo incidence for at least 1 paroxetine group. |
|||||
| Body as a Whole
Asthenia |
0% |
2.9% |
10.6% |
13.9% |
12.7% |
|
Dermatology
Sweating |
2% |
1% |
6.7% |
8.9% |
11.8% |
|
Gastrointestinal
Constipation Decreased Appetite Diarrhea Dry Mouth Nausea |
5.9% 2% 7.8% 2% 13.7% |
4.9% 2% 9.8% 10.8% 14.7% |
7.7% 5.8% 19.2% 18.3% 26.9% |
9.9% 4% 7.9% 15.8% 34.7% |
12.7% 4.9% 14.7% 20.6% 36.3% |
|
Nervous System
Anxiety Dizziness Nervousness Paresthesia Somnolence Tremor |
0% 3.9% 0% 0% 7.8% 0% |
2% 6.9% 5.9% 2.9% 12.7% 0% |
5.8% 6.7% 5.8% 1% 18.3% 7.7% |
5.9% 8.9% 4% 5% 20.8% 7.9% |
5.9% 12.7% 2.9% 5.9% 21.6% 14.7% |
|
Special Senses
Blurred Vision |
2% |
2.9% |
2.9% |
2% |
7.8% |
|
Urogenital System
Abnormal Ejaculation Impotence Male Genital Disorders |
0% 0% 0% |
5.8% 1.9% 3.8% |
6.5% 4.3% 8.7% |
10.6% 6.4% 6.4% |
13% 1.9% 3.7% |
Adaptation to Certain Adverse Events
Male and Female Sexual Dysfunction With SSRIs
Although changes in sexual desire, sexual performance, and sexual satisfaction often occur as manifestations of a psychiatric disorder, they may also be a consequence of pharmacologic treatment. In particular, some evidence suggests that selective serotonin reuptake inhibitors (SSRIs) can cause such untoward sexual experiences.
Reliable estimates of the incidence and severity of untoward experiences involving sexual desire, performance, and satisfaction are difficult to obtain, however, in part because patients and physicians may be reluctant to discuss them. Accordingly, estimates of the incidence of untoward sexual experience and performance cited in product labeling, are likely to underestimate their actual incidence.
Table 6. Incidence of Sexual Adverse Events in Controlled Clinical Trials
| Paroxetine | Placebo | |
|---|---|---|
|
n (males)
|
1446
|
1042
|
| Decreased Libido |
6-15% |
0-5% |
| Ejaculatory Disturbance |
13-28% |
0-2% |
| Impotence |
2-9% |
0-3% |
|
n (females)
|
1822
|
1340
|
| Decreased Libido |
0-9% |
0-2% |
| Orgasmic Disturbance |
2-9% |
0-1% |
Paroxetine treatment has been associated with several cases of priapism. In those cases with a known outcome, patients recovered without sequelae.
While it is difficult to know the precise risk of sexual dysfunction associated with the use of SSRIs, physicians should routinely inquire about such possible side effects.
Weight and Vital Sign Changes
ECG Changes
Liver Function Tests
Hallucinations
Other Events Observed During the Premarketing Evaluation of Paroxetine
During its premarketing assessment in major depressive disorder, multiple doses of paroxetine were administered to 6,145 patients in phase 2 and 3 studies. The conditions and duration of exposure to paroxetine varied greatly and included (in overlapping categories) open and double-blind studies, uncontrolled and controlled studies, inpatient and outpatient studies, and fixed-dose, and titration studies. During premarketing clinical trials in OCD, panic disorder and generalized anxiety disorder, 542, 469, and 735 patients, respectively, received multiple doses of paroxetine. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of untoward events into a smaller number of standardized event categories.
PRECAUTIONS
Body as a Whole
Infrequent:rare:
Cardiovascular System
Frequent:infrequent:rare:
Digestive System
Infrequent:rare:
Endocrine System
Rare:
Hemic and Lymphatic Systems
Infrequent:rare:
Metabolic and Nutritional
Frequent:infrequent:rare:
Musculoskeletal System
Frequent:infrequent:rare:
Nervous System
Frequent:infrequent:rare:
Respiratory System
Infrequent:rare:
Skin and Appendages
Frequent:infrequent:rare:
Special Senses
Frequentinfrequent:rare:
Urogenital System
Infrequent:rare:
Postmarketing Reports
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Physical and Psychologic Dependence
OVERDOSAGE
Human Experience
Overdosage Management
No specific antidotes for paroxetine are known.Treatment should consist of those general measures employed in the management of overdosage with any drugs effective in the treatment of major depressive disorder.
PRECAUTIONS: Drugs Metabolized by Cytochrome CYP2D6
Physicians' Desk Reference
PAROXETINE DOSAGE AND ADMINISTRATION
Major Depressive Disorder
Usual Initial Dosage
Maintenance Therapy
There is no body of evidence available to answer the question of how long the patient treated with paroxetine tablets should remain on it. It is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy. Whether the dose needed to induce remission is identical to the dose needed to maintain and/or sustain euthymia is unknown.
Obsessive Compulsive Disorder
Usual Initial Dosage
Maintenance Therapy
CLINICAL PHARMACOLOGY: Clinical Trials
Panic Disorder
Usual Initial Dosage
Maintenance Therapy
CLINICAL PHARMACOLOGY: Clinical Trials
Generalized Anxiety Disorder
Usual Initial Dosage
Maintenance Therapy
CLINICAL PHARMACOLOGY: Clinical Trials
Special Populations
Treatment of Pregnant Women During the Third Trimester
WARNINGS : Usage in Pregnancy
Dosage for Elderly or Debilitated Patients, and Patients With Severe Renal or Hepatic Impairment
Switching Patients to or From a Monoamine Oxidase Inhibitor
At least 14 days should elapse between discontinuation of an MAOI intended to treat depression and initiation of therapy with paroxetine tablets. Conversely, at least 14 days should be allowed after stopping paroxetine tablets before starting an MAOI antidepressant (see CONTRAINDICATIONS ).
Use of Paroxetine Tablets With Reversible MAOIs Such as Linezolid or Methylene Blue
Do not start paroxetine tablets in a patient who is being treated with linezolid or methylene blue because there is increased risk of serotonin syndrome or NMS-like reactions. In a patient who requires more urgent treatment of a psychiatric condition, non-pharmacological interventions, including hospitalization, should be considered (see CONTRAINDICATIONS ). In some cases, a patient receiving therapy with paroxetine tablets may require urgent treatment with linezolid or methylene blue. If acceptable alternatives to linezolid or methylene blue treatment are not available and the potential benefits of linezolid or methylene blue treatment are judged to outweigh the risks of serotonin syndrome or NMS-like reactions in a particular patient, paroxetine tablets should be stopped promptly, and linezolid or methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome or NMS-like reactions for 2 weeks or until 24 hours after the last dose of linezolid or methylene blue, whichever comes first. Therapy with paroxetine tablets may be resumed 24 hours after the last dose of linezolid or methylene blue (see WARNINGS ).
Discontinuation of Treatment With Paroxetine Tablets
PRECAUTIONS : Discontinuation of Treatment With Paroxetine Tablets
HOW SUPPLIED
Paroxetine Tablets USP, 20 mg
Bottles of 15 NDC 16590-181-15
Bottles of 28 NDC 16590-181-28
Bottles of 30 NDC 16590-181-30
Bottles of 60 NDC 16590-181-60
Bottles of 90 NDC 16590-181-90
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Manufactured by:
Aurobindo Pharma LLC
Dayton, NJ08810
Manufactured for:
Aurobindo Pharma USA, Inc.
Dayton, NJ08810
Revised: 08/2011
Medication Guide
Paroxetine Tablets, USP
Read the Medication Guide that comes with paroxetine tablets before you start taking them and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment. Talk with your healthcare provider if there is something you do not understand or want to learn more about.
What is the most important information I should know about paroxetine tablets?
Paroxetine tablets and other antidepressant medicines may cause serious side effects, including:
1. Suicidal thoughts or actions:
- Paroxetine tablets and other antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment or when the dose is changed.
- Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions.
- Watch for these changes and call your healthcare provider right away if you notice:
- New or sudden changes in mood, behavior, actions, thoughts, or feelings, especially if severe.
- Pay particular attention to such changes when paroxetine tablets are started or when the dose is changed.
Keep all follow-up visits with your healthcare provider and call between visits if you are worried about symptoms.
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency, especially if they are new, worse, or worry you:
- attempts to commit suicide
- acting on dangerous impulses
- acting aggressive or violent
- thoughts about suicide or dying
- new or worse depression
- new or worse anxiety or panic attacks
- feeling agitated, restless, angry, or irritable
- trouble sleeping
- an increase in activity or talking more than what is normal for you
- other unusual changes in behavior or mood
Call your healthcare provider right away if you have any of the following symptoms, or call 911 if an emergency. Paroxetine tablets may be associated with these serious side effects:
2. Serotonin Syndrome or Neuroleptic Malignant Syndrome-like reactions. This condition can be life-threatening and may include:
- agitation, hallucinations, coma, or other changes in mental status
- coordination problems or muscle twitching (overactive reflexes)
- racing heartbeat, high or low blood pressure
- sweating or fever
- nausea, vomiting, or diarrhea
- muscle rigidity
3. Severe allergic reactions:
- trouble breathing
- swelling of the face, tongue, eyes, or mouth
- rash, itchy welts (hives), or blisters, alone or with fever or joint pain
4. Abnormal bleeding: Paroxetine tablets and other antidepressant medicines may increase your risk of bleeding or bruising, especially if you take the blood thinner warfarin (Coumadin®, Jantoven®), a non-steroidal anti-inflammatory drug (NSAIDs, like ibuprofen or naproxen), or aspirin.
5. Seizures or convulsions
6. Manic episodes:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
7. Changes in appetite or weight.
8. Low salt (sodium) levels in the blood. Elderly people may be at greater risk for this. Symptoms may include:
- headache
- weakness or feeling unsteady
- confusion, problems concentrating or thinking, or memory problems
Do not stop paroxetine tablets without first talking to your healthcare provider. Stopping paroxetine tablets too quickly may cause serious symptoms including:
- anxiety, irritability, high or low mood, feeling restless, or changes in sleep habits
- headache, sweating, nausea, dizziness
- electric shock-like sensations, shaking, confusion
What are paroxetine tablets?
Paroxetine tablets are a prescription medicine used to treat depression. It is important to talk with your healthcare provider about the risks of treating depression and also the risks of not treating it. You should discuss all treatment choices with your healthcare provider. Paroxetine tablets are also used to treat:
- Major Depressive Disorder (MDD)
- Obsessive Compulsive Disorder (OCD)
- Panic Disorder
- Generalized Anxiety Disorder (GAD)
Talk to your healthcare provider if you do not think that your condition is getting better with treatment using paroxetine tablets.
Who should not take paroxetine tablets?
- are allergic to paroxetine or any of the ingredients in paroxetine tablets. See the end of this Medication Guide for a complete list of ingredients in paroxetine tablets.
- take a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- Do not take an MAOI within 2 weeks of stopping paroxetine tablets unless directed to do so by your physician.
- Do not start paroxetine tablets if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your physician.
- People who take paroxetine tablets close in time to an MAOI may have serious or even life-threatening side effects. Get medical help right away if you have any of these symptoms:
- high fever
- uncontrolled muscle spasms
- stiff muscles
- rapid changes in heart rate or blood pressure
- confusion
- loss of consciousness (pass out)
- take MELLARIL® (thioridazine). Do not take MELLARIL® together with paroxetine tablets because this can cause serious heart rhythm problems or sudden death.
- take the antipsychotic medicine pimozide (ORAP®) because this can cause serious heart problems.
What should I tell my healthcare provider before taking paroxetine tablets? Ask if you are not sure.
Before starting paroxetine tablets, tell your healthcare provider if you:
- are pregnant, may be pregnant, or plan to become pregnant. There is a possibility that paroxetine tablets may harm your unborn baby, including an increased risk of birth defects, particularly heart defects. Other risks may include a serious condition in which there is not enough oxygen in the baby’s blood. Your baby may also have certain other symptoms shortly after birth. Premature births have also been reported in some women who used paroxetine tablets during pregnancy.
- are breastfeeding. Paroxetine passes into your milk. Talk to your healthcare provider about the best way to feed your baby while taking paroxetine tablets.
- are taking certain drugs such as:
- triptans used to treat migraine headache
- other antidepressants (SSRIs, SNRIs, tricyclics, or lithium) or antipsychotics
- drugs that affect serotonin, such as lithium, tramadol, tryptophan, St. John’s wort
- certain drugs used to treat irregular heart beats
- certain drugs used to treat schizophrenia
- certain drugs used to treat HIV infection
- certain drugs that affect the blood, such as warfarin, aspirin, and ibuprofen
- certain drugs used to treat epilepsy
- atomoxetine
- cimetidine
- fentanyl
- metoprolol
- pimozide
- procyclidine
- tamoxifen
- have liver problems
- have kidney problems
- have heart problems
- have or had seizures or convulsions
- have bipolar disorder or mania
- have low sodium levels in your blood
- have a history of a stroke
- have high blood pressure
- have or had bleeding problems
- have glaucoma (high pressure in the eye)
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Paroxetine tablets and some medicines may interact with each other, may not work as well, or may cause serious side effects.
Your healthcare provider or pharmacist can tell you if it is safe to take paroxetine tablets with your other medicines. Do not start or stop any medicine while taking paroxetine tablets without talking to your healthcare provider first.
If you take paroxetine tablets, you should not take any other medicines that contain paroxetine, including PAXIL CR and PEXEVA® (paroxetine mesylate).
How should I take paroxetine tablets?
- Take paroxetine tablets exactly as prescribed. Your healthcare provider may need to change the dose of paroxetine tablets until it is the right dose for you.
- Paroxetine tablets may be taken with or without food.
- If you miss a dose of paroxetine tablets, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses of paroxetine tablets at the same time.
- If you take too much paroxetine, call your healthcare provider or poison control center right away, or get emergency treatment.
- Do not stop taking paroxetine tablets suddenly without talking to your doctor (unless you have symptoms of a severe allergic reaction). If you need to stop taking paroxetine tablets, your healthcare provider can tell you how to safely stop taking it.
What should I avoid while taking paroxetine tablets?
Paroxetine tablets can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly. You should not drive, operate heavy machinery, or do other dangerous activities until you know how paroxetine tablets affect you. Do not drink alcohol while using paroxetine tablets.
What are possible side effects of paroxetine tablets?
Paroxetine tablets may cause serious side effects, including all of those described in the section entitled “What is the most important information I should know about paroxetine tablets?”
Common possible side effects in people who take paroxetine tablets include:
- nausea
- sleepiness
- weakness
- dizziness
- feeling anxious or trouble sleeping
- sexual problems
- sweating
- shaking
- not feeling hungry
- dry mouth
- constipation
- infection
- yawning
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of paroxetine tablets. For more information, ask your healthcare provider or pharmacist.
CALL YOUR DOCTOR FOR MEDICAL ADVICE ABOUT SIDE EFFECTS. YOU MAY REPORT SIDE EFFECTS TO THE FDA AT 1-800-FDA-1088 or 1-800-332-1088.
How should I store paroxetine tablets?
- Store paroxetine tablets at room temperature between 20° to 25°C (68° to 77°F).
- Keep paroxetine tablets away from light.
- Keep bottle of paroxetine tablets closed tightly.
Keep paroxetine tablets and all medicines out of the reach of children.
General information about paroxetine tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use paroxetine tablets for a condition for which it was not prescribed. Do not give paroxetine tablets to other people, even if they have the same condition. They may harm them.
This Medication Guide summarizes the most important information about paroxetine tablets. If you would like more information, talk with your healthcare provider. You may ask your healthcare provider or pharmacist for information about paroxetine tablets that is written for healthcare professionals.
For more information about paroxetine tablets call 1-866-850-2876.
What are the ingredients in paroxetine tablets?
Active ingredient: paroxetine hydrochloride
Inactive ingredients in tablets: dibasic calcium phosphate dihydrate, lactose monohydrate, sodium starch glycolate, dibasic calcium phosphate anhydrous, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol and polysorbate 80. In addition to this, 10 mg tablet contains D&C Yellow #10 AluminumLake and FD&C Yellow #6 AluminumLake. 20 mg and 40 mg tablets contain D&C Red #30 AluminumLake. 30 mg tablet contains FD&C Blue #2 AluminumLake.
All brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Aurobindo Pharma LLC
Dayton, NJ 08810
Manufactured for:
Aurobindo Pharma USA, Inc.
Dayton, NJ 08810
Revised: 08/2011
Relabeling and Repackaging by
PACKAGE LABEL - PAROXETINE 20mg TABLETS

PAROXETINEPAROXETINE TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||