Paroxetine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
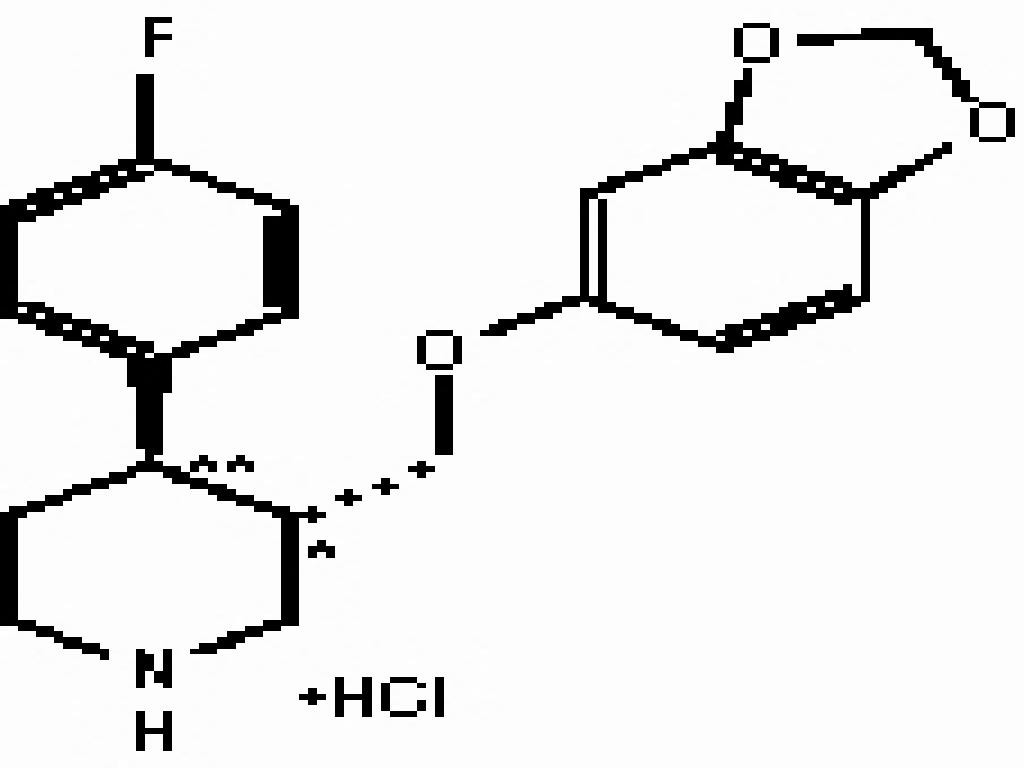
- PAROXETINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PAROXETINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PAROXETINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PAROXETINE DESCRIPTION
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Absorption and Distribution
Metabolism and Excretion
Other Clinical Pharmacology Information
Specific Populations
Renal and Liver Disease
Elderly Patients
Drug-Drug Interactions
Clinical Trials
Major Depressive Disorder
Obsessive Compulsive Disorder
Panic Disorder
Social Anxiety Disorder
Generalized Anxiety Disorder
INDICATIONS & USAGE
Major Depressive Disorder
Obsessive Compulsive Disorder
Panic Disorder
Social Anxiety Disorder
Generalized Anxiety Disorder
PAROXETINE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide RiskScreening Patients for Bipolar Disorder
Potential for Interaction With Monoamine Oxidase Inhibitors
Serotonin Syndrome
Potential Interaction With Thioridazine
Usage in Pregnancy
Teratogenic Effects
Animal Findings
Nonteratogenic Effects
PRECAUTIONS
GeneralActivation of Mania/Hypomania
Seizures
Discontinuation of Treatment With Paroxetine
Akathisia
Hyponatremia
Abnormal Bleeding
Use in Patients With Concomitant Illness
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
Drugs That Interfere With Hemostasis (NSAIDs, Aspirin, Warfarin, etc.)
Interference With Cognitive and Motor Performance
Completing Course of Therapy
Concomitant Medication
Alcohol
Pregnancy
Nursing
LABORATORY TESTS
DRUG INTERACTIONS
TryptophanMonoamine Oxidase Inhibitors
Pimozide
Serotonergic Drugs
Thioridazine
Warfarin
Triptans
Drugs Affecting Hepatic Metabolism
Cimetidine
Phenobarbital
Phenytoin
Drugs Metabolized by CYP2D6
Drugs Metabolized by Cytochrome CYP3A4
Tricyclic Antidepressants (TCAs)
Drugs Highly Bound to Plasma Protein
Drugs That Interfere With Hemostasis (NSAIDs, Aspirin, Warfarin, etc.)
Alcohol
Lithium
Digoxin
Diazepam
Procyclidine
Beta-Blockers
Theophylline
Fosamprenavir/Ritonavir
Electroconvulsive Therapy (ECT)
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Pregnancy Category DLABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
Geriatric Use
PAROXETINE ADVERSE REACTIONS
Associated With Discontinuation of TreatmentCommonly Observed Adverse Events
Major Depressive Disorder
Obsessive Compulsive Disorder
Panic Disorder
Social Anxiety Disorder
Generalized Anxiety Disorder
Incidence in Controlled Clinical Trials
Major Depressive Disorder
Obsessive Compulsive Disorder, Panic Disorder, and Social Anxiety Disorder
Generalized Anxiety Disorder
Dose Dependency of Adverse Events
Adaptation to Certain Adverse Events
Male and Female Sexual Dysfunction With SSRIs
Weight and Vital Sign Changes
ECG Changes
Liver Function Tests
Hallucinations
Other Events Observed During the Premarketing Evaluation of Paroxetine
Body as a Whole
Cardiovascular System
Digestive System
Endocrine System
Hemic and Lymphatic Systems
Metabolic and Nutritional
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Postmarketing Reports
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychologic Dependence
OVERDOSAGE
Human ExperienceOverdosage Management
DOSAGE & ADMINISTRATION
Major Depressive DisorderUsual Initial Dosage
Maintenance Therapy
Obsessive Compulsive Disorder
Usual Initial Dosage
Maintenance Therapy
Panic Disorder
Usual Initial Dosage
Maintenance Therapy
Social Anxiety Disorder
Usual Initial Dosage
Maintenance Therapy
Generalized Anxiety Disorder
Usual Initial Dosage
Maintenance Therapy
Special Populations
Treatment of Pregnant Women During the Third Trimester
Dosage for Elderly or Debilitated Patients, and Patients With Severe Renal or Hepatic Impairment
Switching Patients to or From a Monoamine Oxidase Inhibitor
Discontinuation of Treatment With Paroxetine
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Antidepressant Medicines, Depression and other Serious Mental Illnesses, andSuicidal Thoughts or Actions
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
● What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
-
●
-
●
-
● Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● What else do I need to know about antidepressant medicines?
-
●
-
●
-
●
-
●
-
● This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

ParoxetineParoxetine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!