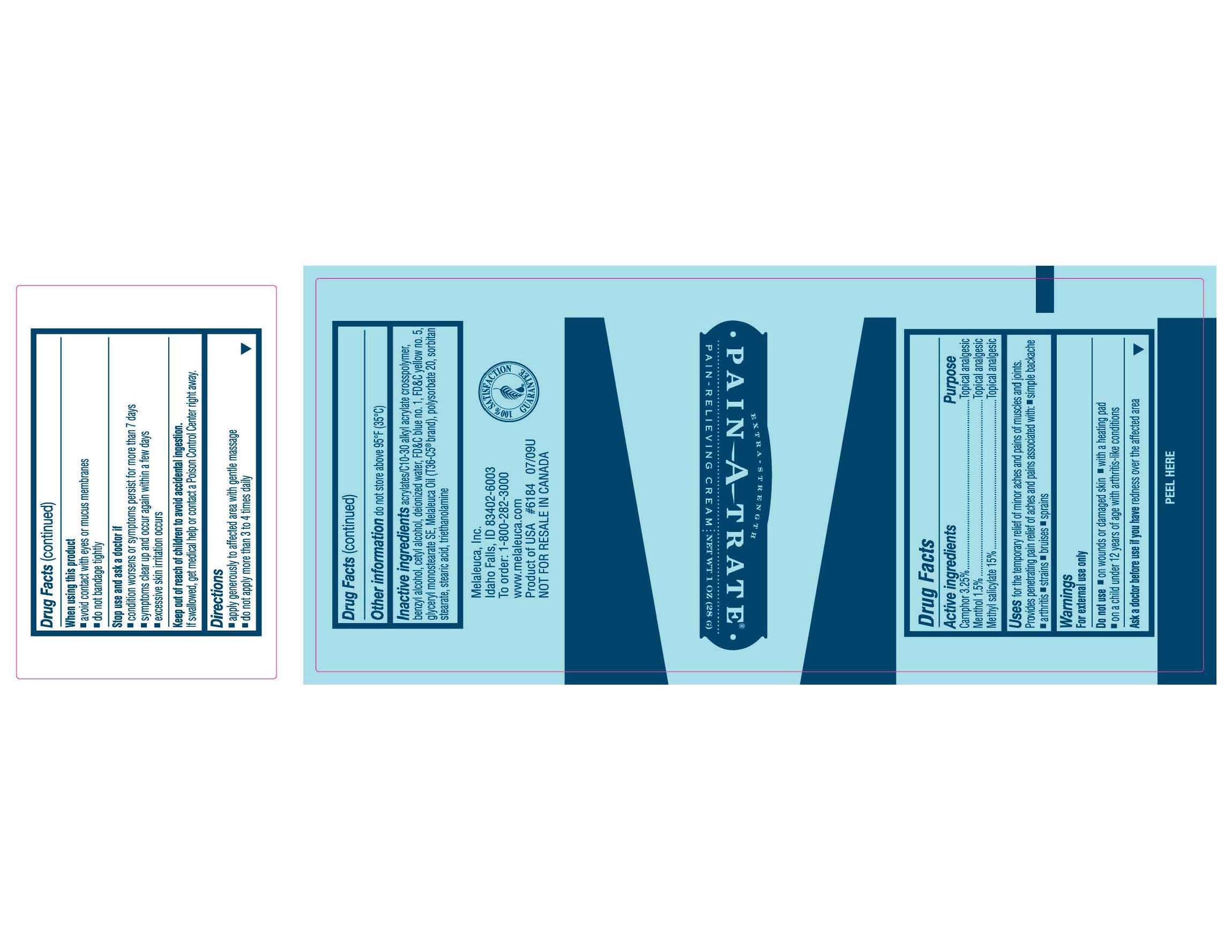
Pain-A-Trate
Pain-A-Trate Extra Strength Pain-Relieving Cream Content of Label
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients
Camphor 3.25%
Menthol, 1.5%
Methyl Salicylate, 15%
Purpose
Purpose
Topical analgesic
Uses
Uses for the temporary relief of minor aches and pains of muscles and joints. Provides penetrating pain relief of aches and pains associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
Do not use
- on wounds or damaged skin
- with a heating pad
- on a child under 12 years of age with arthritis-like conditions
Ask a doctor before use if you have redness over the affected area.
When using this product
- avoid contact with eyes or mucus membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- excessive skin irritation occurs
Keep out of reach of children to avoid accidental ingestion. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply generously to affected area with gentle massage
- do not apply more than 3 to 4 times daily
Other information do not store above 95°F (35°C)
Inactive ingredients acrylates/C10-30 alkyl acrylate crosspolymer, benzyl alcohol, cetyl alcohol, deionized water, FD and C blue no. 1, FD and C yellow no. 5, glyceryl monostearate SE, Melaleuca Oil (T36-C5® brand), polysorbate 20, sorbitan stearate, steric acid, triethanolamine

Pain-A-TrateCamphor and Menthol and Methyl Salicylate CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||