Own
Own BREAKOUT CONTROL Shineless Face Lotion
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Own Uses
- Warnings
- Directions
- Inactive Ingredients
- Questions or Comments?
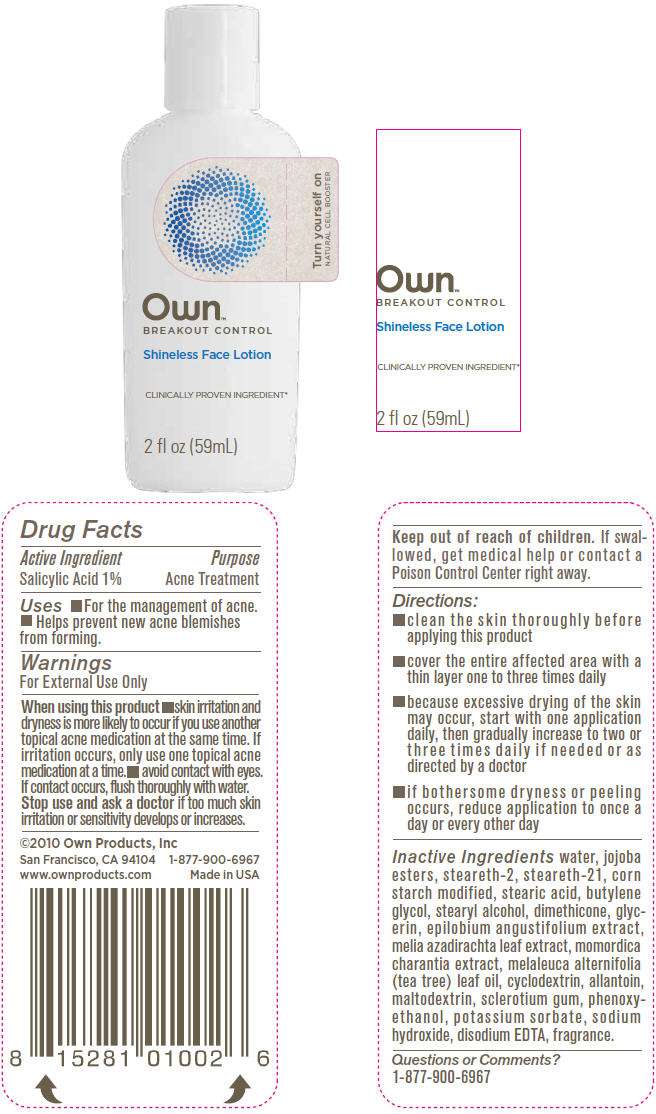
- PRINCIPAL DISPLAY PANEL - 59mL Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Salicylic Acid 1%
Purpose
Acne Treatment
Own Uses
- For the management of acne.
- Helps prevent new acne blemishes from forming.
Warnings
For External Use Only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid contact with eyes. If contact occurs, flush thoroughly with water.
Stop use and ask a doctor if too much skin irritation or sensitivity develops or increases.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive Ingredients
water, jojoba esters, steareth-2, steareth-21, corn starch modified, stearic acid, butylene glycol, stearyl alcohol, dimethicone, glycerin, epilobium angustifolium extract, melia azadirachta leaf extract, momordica charantia extract, melaleuca alternifolia (tea tree) leaf oil, cyclodextrin, allantoin, maltodextrin, sclerotium gum, phenoxyethanol, potassium sorbate, sodium hydroxide, disodium EDTA, fragrance.
Questions or Comments?
1-877-900-6967
PRINCIPAL DISPLAY PANEL - 59mL Bottle Label
Turn yourself on
NATURAL CELL BOOSTER
Own
™
BREAKOUT CONTROL
Shineless Face Lotion
CLINICALLY PROVEN INGREDIENT*
2 fl oz (59mL)
OwnSalicylic Acid LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||