Orajel Instant Relief for Teething Pain
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredientsPurpose
PurposeUses
USEWarnings
Allergy Alert:
Do not use
- more than directed
- for more than 7 days unless directed by a physician or healthcare provider
Stop use and ask a doctor if
- sore mouth symptoms do not improve in 7 days,
- irritation, pain or redness does not go away,
- swelling, rash or fever develops
Keep out of reach of children.
Directions
- wash hands
- cut open tip of tube on score mark
- use your fingertip or cotton applicator to apply a small pea-size amount of Orajel and spread over the gums
- apply to the affected area up to 4 times daily or as directed by a physician or healthcare provider
- for children under 2 years of age, consult a physician or healthcare provider
Principal Display Panel
No 1 TEETHING BRAND
used by Pediatricians
Orajel
Instant Relief
for Teething Pain
cherry flavored gel
LONGER LASTING
NET WT 0.33 OZ (9.4g) Gel ORAL PAIN RELIEVER FOR TEETHING BENZOCAINE 7.5%
Carton image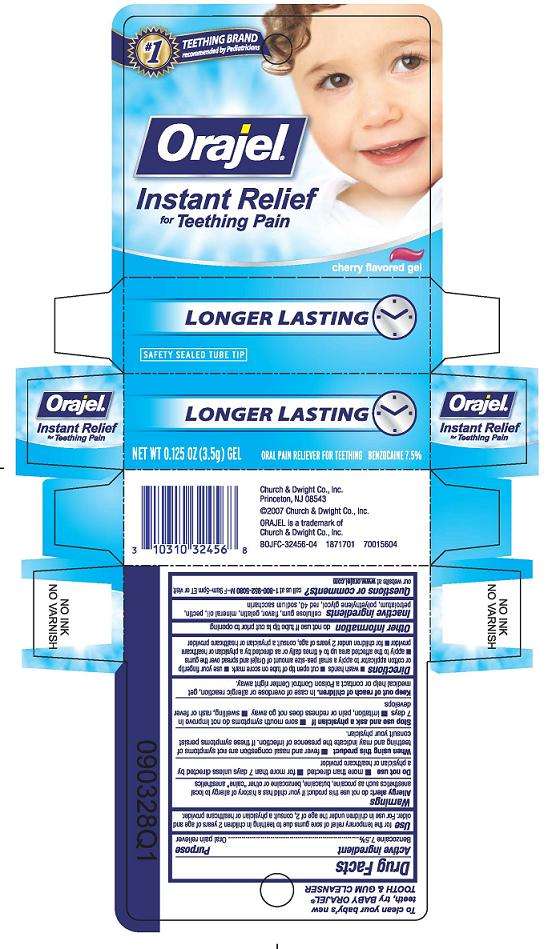
Orajel Instant Relief for Teething PainBenzocaine GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||