NU-DERM PHYSICAL UV BLOCK
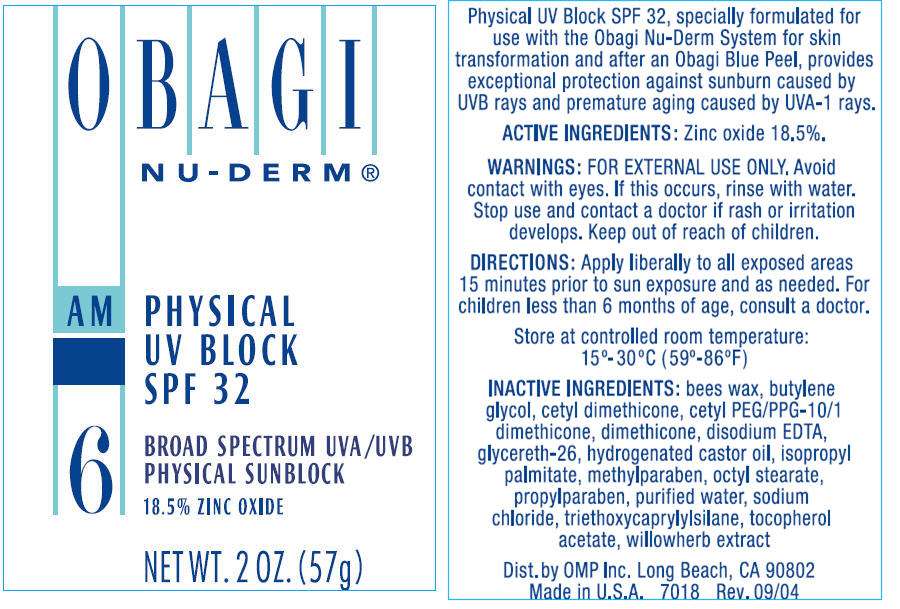
OBAGI NU-DERM® PHYSICAL UV BLOCK SPF 32
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
Zinc oxide 18.5%.
WARNINGS
FOR EXTERNAL USE ONLY. Avoid contact with eyes. If this occurs, rinse with water.
Stop use and contact a doctor if rash or irritation develops.
Keep out of reach of children.
DIRECTIONS
Apply liberally to all exposed areas 15 minutes prior to sun exposure and as needed. For children less than 6 months of age, consult a doctor.
Store at controlled room temperature:
15°-30°C (59°-86°F)
INACTIVE INGREDIENTS
bees wax, butylene glycol, cetyl dimethicone, cetyl PEG/PPG-10/1 dimethicone, dimethicone, disodium EDTA, glycereth-26, hydrogenated castor oil, isopropyl palmitate, methylparaben, octyl stearate, propylparaben, purified water, sodium chloride, triethoxycaprylylsilane, tocopherol acetate, willowherb extract
Dist. by OMP Inc. Long Beach, CA 90802
PRINCIPAL DISPLAY PANEL - 57g Bottle Label
OBAGI
NU-DERM®
AM
PHYSICAL
UV BLOCK
SPF 32
6
BROAD SPECTRUM UVA/UVB
PHYSICAL SUNBLOCK
18.5% ZINC OXIDE
NET WT. 2 OZ. (57g)
NU-DERM PHYSICAL UV BLOCKZINC OXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||