NeoStrata Antibacterial Facial Cleanser
NeoStrata Antibacterial Facial Cleanser
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- NeoStrata Antibacterial Facial Cleanser Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
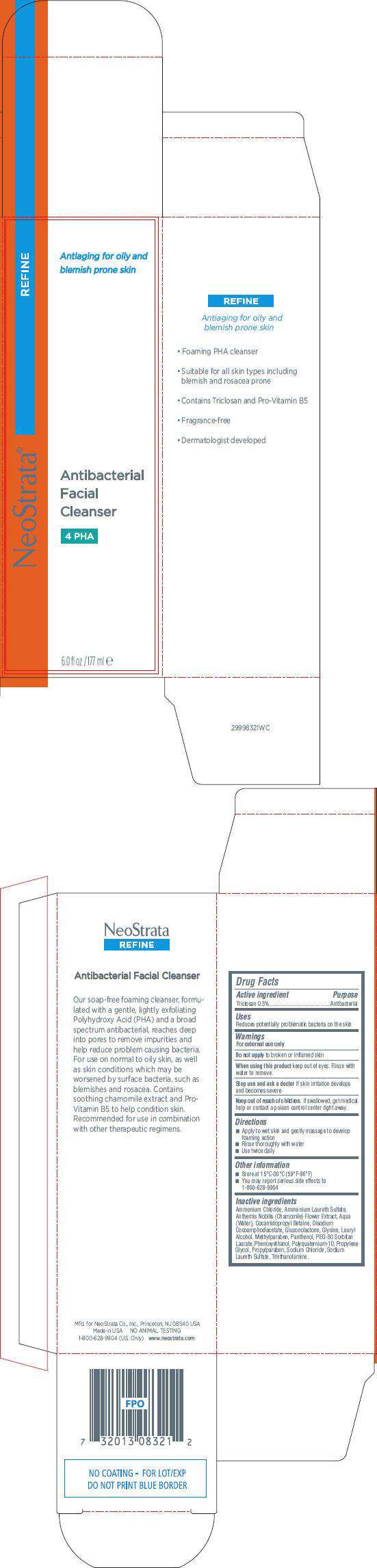
- PRINCIPAL DISPLAY PANEL - 177ml Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Triclosan 0.3%
Purpose
Antibacterial
NeoStrata Antibacterial Facial Cleanser Uses
Reduces potentially problematic bacteria on the skin
Warnings
For external use only
Do not apply to broken or inflamed skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if skin irritation develops and becomes severe.
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Directions
- Apply to wet skin and gently massage to develop foaming action
- Rinse thoroughly with water
- Use twice daily
Other Information
- Store at 15°C-30°C (59°F-86°F)
- You may report serious side effects to 1-800-628-9904
Inactive Ingredients
Ammonium Chloride, Ammonium Laureth Sulfate, Anthemis Noblis (Chamomile) Flower Extract, Aqua (Water), Cocamidopropyl Betaine, Disodium Cocoamphodiacetate, Gluconolactone, Glycine, Lauryl Alcohol, Methylparaben, Panthenol, PEG-80 Sorbitan Laurate, Phenoxyethanol, Polyquaternium-10, Propylene Glycol, Propylparaben, Sodium Chloride, Sodium Laureth Sulfate, Triethanolamine.
PRINCIPAL DISPLAY PANEL - 177ml Carton
NeoStrata ®
REFINE
Antiaging for oily and
blemish prone skin
Antibacterial
Facial
Cleanser
4 PHA
6.0 fl oz / 177 ml e
NeoStrata Antibacterial Facial CleanserTRICLOSAN LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||