Nefazodone Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- NEFAZODONE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- NEFAZODONE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- NEFAZODONE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
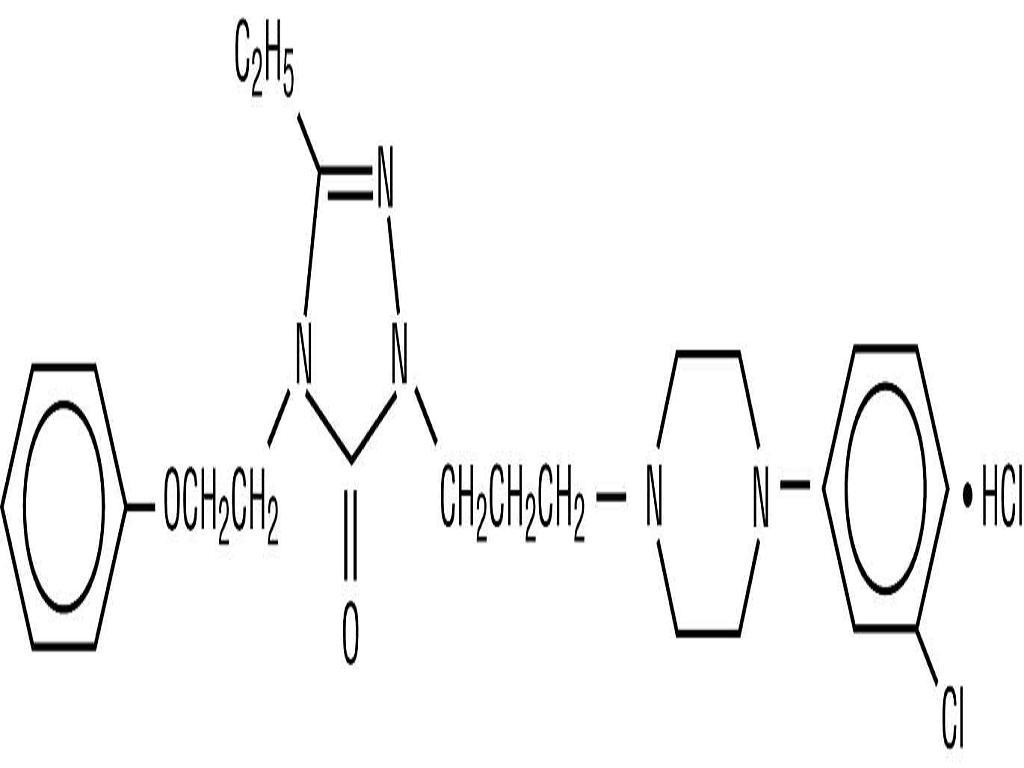
NEFAZODONE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
Distribution
Protein Binding
Effect of Food
Renal Disease
Liver Disease
Age/Gender Effects
Clinical Efficacy Trial Results
Studies in Outpatients With Depression
Studies inInpatients
Studies ofRelapse Prevention in Patients Recently Recovered (Clinically) From Depression
Comparisons of Clinical Trial Results
Uses
INDICATIONS AND USAGENEFAZODONE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide RiskScreening Patients for Bipolar Disorder
Hepatotoxicity
Potential for Interaction With Monoamine Oxidase Inhibitors
Interaction With Triazolobenzodiazepines
Triazolam
Alprazolam
Potential Terfenadine, Astemizole, Cisapride, and Pimozide Interactions
Interaction With Carbamazepine
PRECAUTIONS
GeneralHepatotoxicity
Postural Hypotension
Activation of Mania/Hypomania
Seizures
Priapism
Use in Patients With Concomitant Illness
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
Hepatotoxicity
Time to Response/Continuation
Interference With Cognitive and Motor Performance
Pregnancy
Nursing
Concomitant Medication
Alcohol
Allergic Reactions
Visual Disturbances
LABORATORY TESTS
DRUG INTERACTIONS
Drugs Highly Bound to Plasma ProteinCNS-Active Drugs
Cimetidine
Theophylline
Cardiovascular-Active Drugs
Immunosuppressive Agents
Pharmacokinetics of Nefazodone inPoor Metabolizers'and Potential Interaction With Drugs That Inhibit and/or Are Metabolized by Cytochrome P450 Isozymes
Electroconvulsive Therapy (ECT)
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Teratogenic EffectsPregnancy category C
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
Geriatric Use
NEFAZODONE HYDROCHLORIDE ADVERSE REACTIONS
Associated With Discontinuation of TreatmentIncidence in Controlled Trials
Commonly Observed Adverse Events in Controlled Clinical Trials
Adverse Events Occurring at an Incidence of 1% or More Among Nefazodone-Treated Patients
Dose Dependency of Adverse Events
*
*
Visual Disturbances
Vital Sign Changes
Weight Changes
Laboratory Changes
ECG Changes
Other Events Observed During the Premarketing Evaluation of Nefazodone
Postintroduction Clinical Experience
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceOverdosage Management
DOSAGE & ADMINISTRATION
Initial Treatment
Dosage for Elderly or Debilitated Patients
Maintenance/Continuation/Extended Treatment
Switching Patients to or From a Monoamine Oxidase Inhibitor
HOW SUPPLIED
REFERENCES
INFORMATION FOR PATIENTS
-
● Yellowing of the skin or whites of eyes (jaundice)
-
● Unusually dark urine
-
● Loss of appetite that lasts several days or longer
-
● Nausea
-
● Abdominal (lower stomach) pain
-
● People who currently have liver problems should not take nefazodone.
-
● are taking Seldane(pimozide), used to treat Tourette(carbamazepine), used to control seizures.
-
● currently have liver problems.
-
● are taking or have taken within the last 14 days one of the medicines for depression known as monoamine oxidase inhibitors (MAOIs), such as Nardilor Parnate
-
● Be sure to tell your doctor if you
-
● are taking any other medicine, vitamin supplement, or herbal remedy, including those sold without a prescription (over-the-counter);
-
● have heart problems or have had a heart attack or stroke;
-
● have had manic episodes (extreme agitation or excitability);
-
● have ever attempted suicide;
-
● have had convulsions (seizures);
-
● are pregnant or breast-feeding.
-
● How should I take nefazodone?
-
● It may take a while for you to feel that nefazodone is working. You may not feel the full effect for several weeks. Once you feel better, it is important to keep taking nefazodone as directed by your doctor.
-
● If you miss a dose of nefazodone, skip that dose and continue with your regular schedule. Never take 2 doses at the same time.
-
● If you think that you have taken more nefazodone than prescribed, contact your doctor, local poison control center, or emergency room right away.
-
● What should I avoid while taking nefazodone?
-
● Before taking nefazodone, tell your doctor about any medicines you are taking, including vitamin supplements, herbal remedies, and any non-prescription (over-the-counter) medicines. Some of these medicines may affect how nefazodone works and should not be used in combination without talking to your doctor.
-
● Do not drink alcoholic beverages while taking nefazodone.
-
● Tell your doctor if you are pregnant, planning to become pregnant, or become pregnant while taking nefazodone. It is not known whether nefazodone can harm your unborn baby.
-
● Talk with your doctor before taking nefazodone if you are breast-feeding. It is not known whether nefazodone can pass through your breast milk to the baby.
-
● What are the possible side effects of nefazodone?
-
● Unusually dark urine
-
● Loss of appetite that lasts several days or longer
-
● Severe nausea
-
● Abdominal (lower stomach) pain
-
● Rash or hives
-
● Seizure (convulsion)
-
● Fainting
-
● Erection that lasts too long
-
● Tell your doctor right away about any side effects that you have or discomfort that you experience. Do not change your dose or stop taking nefazodone without talking with your doctor first.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Nefazodone HydrochlorideNefazodone Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!